Abstract
Purpose
Artesunate–amodiaquine (AS–AQ) and artemether–lumefantrine (AL) have been widely used for the treatment of uncomplicated Plasmodium falciparum malaria since 2005 in Gabon. Since 2011, a rebound of malaria morbidity has been observed in this country, while no survey evaluating ACT efficacy was performed. During the same period, parasite resistance against artemisinin has been reported in Asia. The aim of this study was to assess the efficacy and tolerability of these two drugs in two sentinel sites of Gabon 10 years after their implementation.
Methods
Children aged from 12 to 144 months with uncomplicated malaria were recruited at the Regional Hospital of Melen, Libreville and in the Urban Health Center of Franceville between March 2014 and September 2015. The therapeutic efficacy was evaluated according to the WHO 2008 protocol of 28-day follow-up and PCR-uncorrected/corrected treatment outcomes were assessed.
Results
One hundred and eighty-five children (98 ASAQ and 89 AL) were followed up until day 28. The PCR-corrected ACPR was 98.9% for AS–AQ and 96.4% for AL. Late therapeutic failure rate was 3.6% and 1.1% for AL and AS–AQ, respectively (p = 0.2). Adverse events and serious adverse events were rarely observed with both treatments.
Conclusion
AS–AQ and AL are still efficacious and well-tolerated for the treatment of uncomplicated malaria in Gabonese children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 2000, the majority of malaria-endemic countries substituted monotherapies by artemisinin-based combination therapies (ACTs) for uncomplicated malaria treatment [20]. Following ACTs use, a reduction of malaria mortality by 62% globally between 2000 and 2015, and by 29% between 2010 and 2015 was estimated [23]. Resistance to artemisinin and its spread are now reported in South-East Asia. This region is known to have been focus of origin of antimalarial drug resistance before it reaches Africa [2, 6].
In Gabon, malaria is the second leading cause of hospitalization in pediatrics wards after respiratory tract infections; it is responsible for more than one-third of all febrile patients [4]. Almost all cases are due to P. falciparum, of which strains with molecular markers of artemisinin partner drugs are highly frequent in different areas of the country [11, 13].
At the time of artesunate–amodiaquine (AS–AQ) and artemether–lumefantrine (AL) implementation in public health center as the first- and second-line treatments for uncomplicated malaria (2003–2005), respectively, their efficacy was estimated at 99–100% [1, 3]. However, reports from sentinel sites for malaria surveillance highlighted a reduced AS–AQ prescription in health centers, frequent stock outs of AL and an increase of malaria prevalence, 7–10 years later [14]. In 2013, the national recommendations for uncomplicated malaria treatment changed and both drugs were adopted as first-line treatments, while dihydroartemisinin–piperaquine (DHAP) was selected as the second-line treatment [14].
This study presents the results of a clinical trial evaluating AS–AQ and AL efficacy and tolerability in two cities of Gabon (Libreville and Franceville), where both molecular markers of resistance as well as a rebound of malaria morbidity have been previously described [8, 12].
Materials and Methods
Study Sites and Procedure of Patients’ Screening
This prospective study was carried out in two sentinel sites for malaria surveillance between March 2014 and September 2015 in Gabon. Precisely, the study sites were the Operational and Clinical Research Unit (OCRU) of the Regional Hospital of Melen (RHM), which is located at 11 km from Libreville (West part of Gabon) and the Urban Health Center of Franceville (south-east region of the country). In both cities, malaria transmission is perennial, with an entomological inoculation rate estimated at 33.9 infected bites per person per year [15]. P. falciparum represents more than 93% of the parasite species diagnosed [12, 15].
During the study period, children aged from 12 to 144 months with the following criteria were approached: febrile (tympanic temperature of > 37.5 °C) or with a 24–48 h history of fever before the day of consultation, ability to swallow oral medication, willingness to comply with the duration of the study, no signs of complicated malaria as per WHO guidelines [21], ability to attend the outpatient clinic on stipulated days for the follow-up, and parent or guardian written informed consent for the child participation in the study. Children were first screened for malaria using the SD Bioline Pf/pan rapid diagnosis test RDT [5]. In case of RDTs’ positivity, thick and thin blood smears were performed to determine the parasite density and to identify the species [16]. Each child was included if he/she had a P. falciparum mono infection with a parasite count between 2000 and 200,000 asexual forms per microliter of blood (p/µL). Criteria of exclusion were the presence of complicated malaria as defined by WHO 2000 [19], a mixed Plasmodium infection, concomitant disease, or other danger signs, known allergy to AS–AQ and AL, presence of a treatment with an antimalarial drug in the previous 3 weeks.
Treatment
After enrollment, participants received appropriate dose of AL (Coartem® Novartis) and AS–AQ (Coarsucam®, Sanofi Aventis) at the study site during three consecutive days. They were observed within 1 h after administration. Children who vomited less than 30 min after drug administration were given a second dose and were observed for 30 min more. They were monitored according to the 28-day-WHO protocol (WHO 2003).
For each patient, the outcome of the treatment was classified based on clinical and parasitological tests as early treatment failure (ETF), late treatment failure (LTF), late clinical failure (LCF), late parasitologic failure (LPF), and adequate clinical and parasitological response (ACPR) as per WHO, 2003 protocol [22].
In case of therapeutic failure, patients were retreated with dihydroartemisinin–piperaquine phosphate combination (DHAP). Recrudescence and reinfection cases were distinguished using merozoite surface protein 1 (block 2) and 2 (block 3) gene genotyping by PCR as previously described [18].
Statistical Analysis
All data were entered into an Excel-based program and analyzed using the computer program developed by WHO for antimalarial drug efficacy. This software includes formula for the classification of treatment outcome with and without PCR. Patients who were excluded or withdrew from the study were not included in the per protocol analysis.
Ethical Consideration
The study was conducted in accordance with the principles of good clinical practices (GCP) and reviewed and approved by Gabonese National Ethics Committee and the World Health Organization (WHO) Ethical Review Committee. A written informed consent was obtained from parents or guardians of all study participants.
Results
Study Population Characteristics
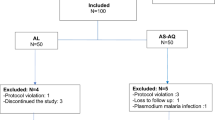
A total of 222 of the 1425 screened children fulfilled the inclusion criteria. Among them, 116 patients received AS–AQ and 106 AL. The excluded patients during the follow-up represented 17.3% (n = 39/225): 17 from the AL group vs 22 from AS–AQ. The reasons for exclusion are shown in the trial profile (Fig. 1). The mean temperature [34.4 °C (± 1.2) vs 38.5 °C (± 1.3)] and the median density parasite (20,542 [1715–179,000] p/µL vs 27,941 [1127–198,800] p/µL) were significantly higher in the AS–AQ group compared to AL, respectively (p = 0.04).
Treatment Outcome
In both groups, the parasite clearance was obtained from the second day following the first dose administration. The results of the treatment efficacy before and after PCR correction are reported in Table 1. After PCR correction, the frequency of ACPR was similar between AS–AQ (98.9%, n = 89) and AL (96.4%, n = 82) treatment groups (p = 0.3).
The microscopic gametocytemia was cleared from day 1 for AL and day 3 for AS–AQ-treated children.
Tolerability and Safety
All the recruited patients recovered rapidly during the first 72 h of follow-up (Fig. 2). Minor and serious adverse events were rarely observed in AS–AQ vs AL groups, respectively: vomiting 2.0% (n = 2) vs 5.6% (n = 5) (p = 0.2), cough 16.6% (n = 16) vs 6.7% (n = 6) (p = 0.004), asthenia 14.5% (n = 14) vs 5.6% (n = 5) (p = 0.05), and abdominal pain 2.0% (n = 2) vs 2.2 (n = 2) (p = 0.6). Hemoglobinuria (n = 1) and convulsion (n = 1) were found in only one patient treated with AS–AQ and in one child who received AL.
Discussion
10 years after the implementation of AS–AQ and AL for the treatment of uncomplicated malaria in Gabon, it was necessary to re-evaluate the therapeutic efficacy of these combinations. This clinical trial was performed in two sentinel sites for malaria surveillance according to the WHO procedures. The data obtained confirmed that both drugs remain efficacious and well-tolerated in Gabon. Temperature recovery from day 0 to day 28 occurred swiftly in both treated groups as reported by other authors through assessment of these two drugs. Likewise, the occurrence of other adverse events was rare [17, 24].
PCR-corrected cure rates for AL and AS–AQ were similar (96.5%) and remain high and adequate according to WHO recommendations. Others studies reported cure rate varying from 99.3 to 100% after treatment with AS–AQ and from 99.3 to 100% for AL [7, 24]. In the present study, the efficacy rates were obtained when ACTs coverage was below 60% in the country. This clinical trial is the first performed in Franceville, where the frequency of molecular markers associated with the resistance of P. falciparum to AQ and Lumefantrine is non negligible [9]. The relatively and non-significant lower efficacy of AL, in comparison with AS–AQ and also to the previous historical estimates, could be a first signal of a potential decline of AL parasite effect. However, fever and microscopic gametocytemia clearance tended to be longer among patients treated with AS–AQ, compared to AL. The efficacy of the AS–AQ combination on gametocytes clearance has been reported by other authors [10]. Both ACTs appeared to be safe and well-tolerated, although vomiting events were associated with the use of AL in five patients.
AL and AS–AQ are still efficacious and well-tolerated in Gabon. Their use as first-line treatment is not compromised. Continuous monitoring throughout the country is necessary to allow early detection of decline efficacy of both drugs.
Change history
11 October 2019
Unfortunately two errors appeared in this article.
References
Adjuik M, Agnamey P, Babiker A, Borrmann S, Brasseur P, Cisse M et al (2002) Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomised, multicentre trial. Lancet 359:1365–1372. https://doi.org/10.1016/S0140-6736(02)08348-4
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S et al (2014) Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423
Bouyou-Akotet MK, Nzenze-Afène S, Mabika-Mafoumbi M, Pemba M, Effame Eya E, Kombila M (2006) Evaluation de l’efficacité et de la toléance de l’Arsucam®, de l’artequin®, et du Coartem® dans le traitement du paludisme non compliqué de l’enfant. Bullin Medical d’Owendo 11:70–75
Bouyou-Akotet MK, Offouga CL, Mawili-Mboumba DP, Essola L, Madoungou B, Kombila M et al (2014) Falciparum malaria as an emerging cause of fever in adults living in Gabon, Central Africa. BioMed Res Int. https://doi.org/10.1155/2014/351281
Djallé D, Gody JC, Moyen JM, Tekpa G, Ipero J, Madji N et al (2014) Performance of Paracheck™-Pf, SD Bioline malaria Ag-Pf and SD Bioline malaria Ag-Pf/pan for diagnosis of falciparum malaria in the Central African Republic. BMC Infect Dis 14:109. https://doi.org/10.1186/1471-2334-14-109
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J et al (2009) Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467
Guthmann JP, Cohuet S, Rigutto C, Fortes F, Saraiva N, Kiguli J et al (2006) High efficacy of two artemisinin-based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. Am J Trop Med Hyg 75:143–145. https://doi.org/10.1016/j.meegid.2011.01.003
Lekana-Douki JB, Dinzouna BSD, Zatra R, Zang Edou SE, Ekomy H, Bisvigou U et al (2011) Increased prevalence of the Plasmodium falciparum Pfmdr1 86N genotype among field isolates from Franceville, Gabon after replacement of chloroquine by artemether lumefantrine and artesunate-mefloquine. Infect Genet Evol 11:512–517. https://doi.org/10.1016/j.meegid.2011.01.003
Lekana-Douki JB, Pontarollo J, Zatra R, Toure-Ndouo FS (2011) Malaria in Gabon: results of a clinical and laboratory study at the Chinese–Gabonese Friendship Hospital of Franceville. Cahier Santé 21:193–198. https://doi.org/10.1684/san.2011.0263
Makanga M, Bassat Q, Falade CO, Premji ZG, Krudsood S, Hunt P et al (2011) Efficacy and safety of artemether-lumefantrine in the treatment of acute, uncomplicated Plasmodium falciparum malaria: a pooled analysis. Am J Trop Med Hyg 85:793–804. https://doi.org/10.4269/ajtmh.2011.11-0069
Maghendji-Nzondo S, Kouna LC, Mourembou G, Boundenga L, Imboumy-Limoukou RK, Matsiegui PB et al (2016) Malaria in urban, semi-urban and rural areas of southern of Gabon: comparison of the Pfmdr1 and Pfcrt genotypes from symptomatic children. Malar J 15:420. https://doi.org/10.1186/s12936-016-1469-1
Mawili-Mboumba DP, Bouyou Akotet MK, Kendjo E, Medang MO, Medang MO, Mbina JR et al (2013) Increase in malaria prevalence and age of at risk population in different areas of Gabon’. Malar J 12:3. https://doi.org/10.1186/1475-2875-12-3
Mawili-Mboumba DP, Ndong Ngomo JM, Maboko F, Guiyedi V, Mourou Mbina JR, Kombila M et al (2014) Pfcrt 76Tand pfmdr1 86Yallele frequency in Plasmodium falciparum isolates and use of self-medication in a rural area of Gabon. Trans R Soc Trop Med Hyg 108:729–734. https://doi.org/10.1093/trstmh/tru147
Ministère de la santé (2013) Rapport de l’atelier national de consensus sur les perspectives thérapeutiques du paludisme. Programme national de lutte contre le paludisme, Gabon
Mourou JR, Coffinet T, Jarjaval F, Cotteaux C, Pradines E, Godefroy L et al (2012) Malaria transmission in Libreville: results of a 1 year survey. Malar J 11:40. https://doi.org/10.1186/1475-2875-11-40
Planche T, Krishna S, Kombila M, Engel K, Ngou-Milama E et al (2001) Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg 65:599–602
Schramm B, Valeh P, Baudin E, Mazinda CS, Smith R, Pinoges L et al (2013) Tolerability and safety of artesunate-amodiaquine and artemether-lumefantrine fixed dose combinations for the treatment of uncomplicated Plasmodium falciparum malaria: two open-label, randomized trials in Nimba County, Liberia. Malar J 12:250. https://doi.org/10.1186/1475-2875-12-250
Snounou G, Beck HP (1998) The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitol Today 14:462–467. https://doi.org/10.1016/S0169-4758(98)01340-4
World Health Organisation (1993) Implementation of the global malaria control strategy. Report of a WHO study group on the implementation of the global plan of action for malaria control 1993–2000. World Health Organ Tech Rep Ser 839:1–57
World Health Organisation (2001) Global partnership to roll back malaria. Antimalarial drug combination therapy: report of a WHO technical consultation. World Health Organization, Geneva. http://www.who.int/iris/handle/10665/66952
World Health Organization (2006) Guidelines for the treatment of malaria, 2nd edn. World Health Organization, Geneva. http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf
World Health Organization (2009) World Malaria Report—World Health Organization. www.who.int/malaria/world_malaria_report_2009/en/
World Health Organisation (2016) World Health Organization. World Malaria Report. http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1
Yavo W, Konaté A, Kassi Kondo, Djohan V, Kpongbo Angora E, Kiki-Barro PC et al (2015) Efficacy and safety of artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum Malaria in sentinel sites across Côte d’Ivoire. Malar Res Treat. https://doi.org/10.1155/2015/878132
Acknowledgements
We are grateful to the patients who participated to the study and the Team of the Department of Parasitology–Mycology, Faculty of Medicine.
Author information
Authors and Affiliations
Contributions
JMNN, GJOM, BMD, and CLO were the Investigators. JVKL and NPM’B were involved in the laboratory analysis. PR, MKB-A, and JMNN have performed data analysis and interpretation. JMNN, JBL-D, DPM-M, and MKB-A wrote the draft. MKB-A, TF, and PR conceived the study. JBL-D and DPM-M did the coordination of the study activities.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ndong Ngomo, J.M., Ondzagha Megnie, G.J., Moutombi Ditombi, B. et al. Persistence of High In Vivo Efficacy and Safety of Artesunate–Amodiaquine and Artemether–Lumefantrine as the First- and Second-Line Treatments for Uncomplicated Plasmodium falciparum Malaria 10 Years After Their Implementation in Gabon. Acta Parasit. 64, 898–902 (2019). https://doi.org/10.2478/s11686-019-00115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00115-y