Abstract
Purpose
Metalloproteinases are a key component of the pathogenesis of abdominal hernias. Obesity is considered a risk factor in herniogenesis and hernia recurrence. The aim of this study was to evaluate the serum concentrations of metalloproteinase-2 (MMP-2), MMP-9, MMP-13, and adiponectin in morbidly obese and nonoverweight controls.
Materials and methods
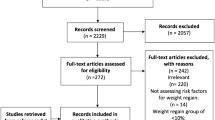
The participants were recruited from among patients undergoing bariatric and non-bariatric surgery and divided into two groups: I (body mass index (BMI)≥35 kg/m2, n=40) and II (BMI<25 kg/m2, n=30). Serum concentrations of MMP-2, MMP-9, MMP-13, and adiponectin were measured using enzyme-linked immunosorbent assay (ELISA).
Results
A statistically significant difference between groups was observed for MMP-2 concentration. The median MMP-9 concentration was higher in the obese group, but the difference was not statistically significant. Median MMP-13 concentrations did not differ between groups. Serum adiponectin concentration was insignificantly higher in the non-obese group.
Conclusions
The elevated serum MMP-2 and MMP-9 concentrations in obese individuals may be related to the higher incidence of incisional hernias in this population.
摘要
目的
主要研究病态肥胖患者与正常人血清中金属蛋白 酶2(MMP-2)、MMP-9、MMP-13 和脂联素的 浓度。
创新点
建立血清中MMP-2、MMP-9、MMP-13 和脂联 素的浓度与肥胖和切口疝的关系。
方法
参与实验的人员为进行肥胖手术的患者和不进行 肥胖手术的患者,并将他们分为两组:I(体重 指数(BMI)≥35 kg/m2,n=40)和II (BMIlt;25 kg/m2, n=30),并使用酶联免疫吸附实验测定受试人员 体内血清中MMP-2、MMP-9、MMP-13 和脂联 素的浓度。
结论
MMP-2 的浓度在肥胖组中更高,且在两组血清 中有显著性差异。虽然MMP-9 的浓度在肥胖组 中更高,但是两组之间没有显著性差异。MMP-13 在两组间没有差异。血清中脂联素的浓度在非肥 胖组更高,但无显著性差异。因此,血清中 MMP-2和MMP-9的浓度在肥胖人群中与更高的 切口疝发病率有关。
Similar content being viewed by others
References
Agren MS, Jorgensen LN, Andersen M, et al., 1998. Matrix metalloproteinase 9 level predicts optimal collagen deposition during early wound repair in humans. Br J Surg, 85(1): 68–71. https://doi.org/10.1046/j.1365-2168.1998.00556.x
Andrade VL, Petruceli E, Belo VA, et al., 2012. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin Biochem, 459(6): 412–415. https://doi.org/10.1016/j.clinbiochem.2012.01.008
Antoniou SA, Antoniou GA, Granderath FA, et al., 2009. The role of matrix metalloproteinases in the pathogenesis of abdominal wall hernias. Eur J Clin Invest, 39(11): 953–959. https://doi.org/10.1111/j.1365-2362.2009.02199.x
Aren A, Gökçe AH, Gökçe FS, et al., 2011. Roles of matrix metalloproteinases in the etiology of inguinal hernia. Hernia, 15(6): 667–671. https://doi.org/10.1007/s10029-011-0846-5
Bellón JM, Bajo A, Ga-Honduvilla N, et al., 2001. Fibroblasts from the transversalis fascia of young patients with direct inguinal hernias show constitutive MMP-2 overexpression. Ann Surg, 233(2): 287–291. https://doi.org/10.1097/00000658-200102000-00020
Bienertová-Vašku J, Novák J, Zlámal F, et al., 2014. The prediction role of indexes of circulating adipokines for common anthropometric and nutritional characteristics of obesity in the obese Central European population. Eat Behav, 15(2): 244–251. https://doi.org/10.1016/j.eatbeh.2014.03.001
Bouloumié A, Sengenès C, Portolan G, et al., 2001. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes, 50(9): 2080–2086. https://doi.org/10.2337/diabetes.50.9.2080
Catalán V, Gómez-Ambrosi J, Ramirez B, et al., 2007. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg, 17(11): 1464–1474. https://doi.org/10.1007/s11695-008-9424-z
Catalán V, Gómez-Ambrosi J, Rodríguez A, et al., 2009. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J Mol Med, 87(8): 803–813. https://doi.org/10.1007/s00109-009-0486-8
Catalán V, Gómez-Ambrosi J, Rodríguez A, et al., 2016. Increased interleukin-32 levels in obesity promote adipose tissue inflammation and extracellular matrix remodeling: effect of weight loss. Diabetes, 65(12): 3636–3648. https://doi.org/10.2337/db16-0287
Derosa G, Ferrari I, D'Angelo A, et al., 2008. Matrix metalloproteinase-2 and-9 levels in obese patients. Endothelium, 15(4): 219–224. https://doi.org/10.1080/10623320802228815
Frühbeck G, 2015. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol, 11(8): 465–477. https://doi.org/10.1038/nrendo.2015.84
Giannakos E, Vardali E, Bartekova M, et al., 2016. Changes in activities of circulating MMP-2 and MMP-9 in patients suffering from heart failure in relation to gender, hypertension and treatment: a cross-sectional study. Physiol Res, 65(Suppl 1): S149–S152.
Glowinska-Olszewska B, Urban M, 2007. Elevated matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in obese children and adolescents. Metabolism, 56(6): 799–805. https://doi.org/10.1016/j.metabol.2007.01.011
Gómez-Ambrosi J, Silva C, Galofré JC, et al., 2011. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring), 19(7): 1439–1444. https://doi.org/10.1038/oby.2011.36
Gumbau V, Bruna M, Canelles E, et al., 2014. A prospective study on inflammatory parameters in obese patients after sleeve gastrectomy. Obes Surg, 24(6): 903–908. https://doi.org/10.1007/s11695-014-1186-1
Gummesson A, Hagg D, Olson FJ, et al., 2009. Adipose tissue is not an important source for matrix metalloproteinase-9 in the circulation. Scand J Clin Lab Invest, 69(6): 636–642. https://doi.org/10.3109/00365510902912747
Henriksen N, 2016. Systemic and local collagen turnover in hernia patients. Dan Med J., 63(7): B5265.
Henriksen NA, Sørensen LT, Jorgensen LN, et al., 2013. Circulating levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with incisional hernia. Wound Repair Regen, 21(5): 661–666. https://doi.org/10.1111/wrr.12071
Jung UJ, Choi MS, 2014. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci, 15(4): 6184–6223. https://doi.org/10.3390/ijms15046184
Klinge U, Si ZY, Zheng H, et al., 2001. Collagen I/III and matrix metalloproteinases (MMP) 1 and 13 in the fascia of patients with incisional hernias. J Invest Surg, 14(1): 47–54. https://doi.org/10.1080/089419301750072202
Liu Y, Min D, Bolton T, et al., 2009. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care, 32(11): 117–119. https://doi.org/10.2337/dc08-0763
Mohebali K, Young DM, Hansen SL, et al., 2009. Open incisional hernia repair at an academic tertiary care medical center. Arch Surg, 144(9): 848–852. https://doi.org/10.1001/archsurg.2009.161
Moreno-Navarrete JM, Catalán V, Whyte L, et al., 2012. The L-a-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes, 61(2): 281–291. https://doi.org/10.2337/db11-0649
Pascual G, Rodríguez M, Gómez-Gil V, et al., 2010. Active matrix metalloproteinase-2 upregulation in the abdominal skin of patients with direct inguinal hernia. Eur J Clin Invest, 40(12): 1113–1121. https://doi.org/10.1111/j.1365-2362.2010.02364.x
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al., 2011. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle, 15(10): 1548–1556.
Rosch R, Klinge U, Si Z, et al., 2002. A role for the collagen I/III and MMP-1/-13 genes in primary inguinal hernia? BMC Med Genet, 3(1): 2. https://doi.org/10.1186/1471-2350-3-2
Salameh JR, Talbott LM, May W, et al., 2007. Role of biomarkers in incisional hernias. Am Surg, 73(6): 561–568.
Smigielski J, Kolomecki K, Ziemniak P, et al., 2009. Degradation of collagen by metalloproteinase 2 in patients with abdominal hernias. Eur Surg Res, 42(2): 118–121. https://doi.org/10.1159/000187643
Smigielski J, Brocki M, Kuzdak K, et al., 2011. Serum MMP 2 and TIMP 2 in patients with inguinal hernias. Eur J Clin Invest, 41(6): 584–588. https://doi.org/10.1111/j.1365-2362.2010.02445.x
Sørensen LT, 2006. Effect of lifestyle, gender and age on collagen formation and degradation. Hernia, 10(6): 456–461. https://doi.org/10.1007/s10029-006-0143-x
Stumpf M, Cao W, Klinge U, et al., 2002. Collagen distribution and expression of matrix metalloproteinases 1 and 13 in patients with anastomotic leakage after largebowel surgery. Langenbecks Arch Surg, 386(7): 502–506. https://doi.org/10.1007/s00423-001-0255-9
Sugerman HJ, KellumJr JM, Reines HD, et al., 1996. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg, 171(1): 80–84. https://doi.org/10.1016/S0002-9610(99)80078-6
Tham JC, Howes N, le Roux CW, 2014. The role of bariatric surgery in the treatment of diabetes. Ther Adv Chronic Dis, 5(3): 149–157. https://doi.org/10.1177/2040622313513313
Trakhtenbroit MA, Leichman JG, Algahim MF, et al., 2009. Body weight, insulin resistance, and serum adipokine levels 2 years after 2 types of bariatric surgery. Am J Med, 122(5): 435–442. https://doi.org/10.1016/j.amjmed.2008.10.035
Tsioufis C, Bafakis I, Kasiakogias A, et al., 2012. The role of matrix metalloproteinases in diabetes mellitus. Curr Top Med Chem, 12(10): 1159–1165. https://doi.org/10.2174/1568026611208011159
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szczęsny, W., Kuligowska-Prusińska, M., Dąbrowiecki, S. et al. Activity of metalloproteinases and adiponectin in obese patients— a possible factor of incisional hernias after bariatric procedures. J. Zhejiang Univ. Sci. B 19, 65–70 (2018). https://doi.org/10.1631/jzus.B1600383
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600383