Abstract
Ce and Mn modified TiO2 sorbents (CeMnTi) were prepared by a co-precipitation method, and their ability to remove elemental mercury from coal gas in a fixed bed reactor was studied. Based on results of Brunauer-Emmett-Teller (BET), X-ray diffraction (XRD), scanning electron microscope (SEM), and X-ray photoelectron spectroscopy (XPS) studies, the modification mechanisms of the CeMnTi sorbents are discussed. Mn doping improved the specific surface area and dispersion of cerium oxides on the sorbent surface, while Ce doping increased the proportion of Mn4+ in manganese oxides by a synergetic effect between manganese oxides and cerium oxides. The effects of the active component, temperature, and coal gas components on the mercury removal performance of the sorbents were investigated. The results showed that the CeMnTi sorbents exhibited high mercury removal efficiency. Ce0.2Mn0.1Ti adsorbed 91.55% elemental mercury from coal gas at 160 °C. H2S and O2 significantly improved the ability of sorbents to remove mercury. Part of the H2S formed stable sulfates or sulfites through a series of oxidation reaction chains on the sorbent surface. HCl also improved the mercury removal performance, but reduced the promotion effect of H2S for mercury removal when coexisting with H2S. CO and H2 had a minor inhibitory effect on mercury adsorption. The recycling performance of the sorbents was investigated by thermal regeneration. The thermal decomposition of the used sorbents indicated that mercury compounds were present mainly in the form of HgO and HgS, and higher temperature was beneficial for regeneration. The formation of sulfates and sulfites in the presence of H2S led to a decrease in mercury removal efficiency.
概要
目的
探明复合金属氧化物吸附剂协同改性机理,研究温度、活性组分负载量和煤气组分等因素对吸附剂脱汞的影响和机理,并进一步通过热脱附再生对吸附剂的再生性能和机理进行探索,为提高该类吸附剂的脱汞效率以及实际工业应用提供理论基础。
创新点
1.利用多种表征方法,推导出了Ce-Mn金属氧化物吸附剂的协同改性机制;2.通过硫化氢存在下的脱汞和再生实验,得出了吸附剂再生后脱汞效率下降的机理:金属硫酸盐和亚硫酸盐的形成使得活性组分出现不可逆损失。
方法
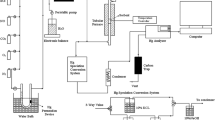
1.通过共沉淀法制备CeMnTi吸附剂;2.在固定床反应器上进行煤气脱汞实验,控制变量为反应温度、活性组分负载量和煤气成分,结合表征方法对脱汞机理进行探究;3.在固定床反应器上通过热脱附再生的方法考察吸附剂再生性能。
结论
Ce0.2Mn0.1Ti吸附剂相对CeTi和MnTi吸附剂具有更高的脱汞效率和热稳定性。其脱汞效率在160°C时达到91.55%,而在200°C时仍保持在83%以上。2. Mn掺杂可改善吸附剂表面积和铈氧化物在吸附剂表面的分散性,而Ce掺杂可通过与锰氧化物的相互作用提高Mn4+的比例。3. H2S可通过在吸附剂表面生成活性硫显著促进汞的吸附,但是H2S也会与活性成分形成硫酸盐和亚硫酸盐,因此很难进行吸附剂的再生。CO和H2的存在会抑制汞的脱除。HCl可提高脱汞性能,而HCl与H2S共存时则会由于竞争吸附使得脱汞效率下降。4. 吸附剂上的汞化合物主要以HgO和HgS的形式存在,且大部分可以在500°C下脱附分解。再生后脱除效率降低的主要原因可能是在H2S存在时形成了硫酸盐和亚硫酸盐。
Similar content being viewed by others
Reference
Andreu N, Flahaut D, Dedryvère R, et al., 2015. XPS investigation of surface reactivity of electrode materials: effect of the transition metal. ACS Applied Materials & Interfaces, 7(12):6629–6636. https://doi.org/10.1021/am5089764
Cheah S, Carpenter DL, Magrini-Bair KA, 2009. Review of mid- to high-temperature sulfur sorbents for desulfurization of biomass- and coal-derived syngas. Energy & Fuels, 23(11):5291–5307. https://doi.org/10.1021/ef900714q
Ding ZY, Li LX, Wade D, et al., 1998. Supercritical water oxidation of NH3 over a MnO2/CeO2 catalyst. Industrial & Engineering Chemistry Research, 37(5):1707–1716. https://doi.org/10.1021/ie9709345
Dong J, Xu ZH, Kuznicki SM, 2009. Mercury removal from flue gases by novel regenerable magnetic nanocomposite sorbents. Environmental Science & Technology, 43(9): 3266–3271. https://doi.org/10.1021/es803306n
Gao X, Jiang Y, Zhong Y, et al., 2010. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3. Journal of Hazardous Materials, 174(1–3): 734–739. https://doi.org/10.1016/j.jhazmat.2009.09.112
Granite EJ, Pennline HW, Hargis RA, 2000. Novel sorbents for mercury removal from flue gas. Industrial & Engineering Chemistry Research, 39(4):1020–1029. https://doi.org/10.1021/ie990758v
He C, Shen BX, Chen JH, et al., 2014. Adsorption and oxidation of elemental mercury over Ce-MnOx/Ti-PILCs. Environmental Science & Technology, 48(14):7891–7898. https://doi.org/10.1021/es5007719
Hou WH, Zhou JS, Qi P, et al., 2014. Effect of H2S/HCl on the removal of elemental mercury in syngas over CeO2-TiO2. Chemical Engineering Journal, 241:131–137. https://doi.org/10.1016/j.cej.2013.12.047
IEA (International Energy Agency), 2019. World Energy Outlook 2019. https://www.iea.org/reports/world-energy-outlook-2019
Jampaiah D, Ippolito SJ, Sabri YM, et al., 2016. Ceria-zirconia modified MnOx catalysts for gaseous elemental mercury oxidation and adsorption. Catalysis Science & Technology, 6(6):1792–1803. https://doi.org/10.1039/c5cy01534k
Ji L, Sreekanth PM, Smirniotis PG, et al., 2008. Manganese oxide/titania materials for removal of NOx and elemental mercury from flue gas. Energy & Fuels, 22(4):2299–2306. https://doi.org/10.1021/ef700533q
Kobayashi M, Flytzani-Stephanopoulos M, 2002. Reduction and sulfidation kinetics of cerium oxide and Cu-modified cerium oxide. Industrial & Engineering Chemistry Research, 41(13):3115–3123. https://doi.org/10.1021/ie010815w
Li HL, Wu CY, Li Y, et al., 2011. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environmental Science & Technology, 45(17):7394–7400. https://doi.org/10.1021/es2007808
Li JF, Yan NQ, Qu Z, et al., 2010. Catalytic oxidation of elemental mercury over the modified catalyst Mn/α-Al2O3 at lower temperatures. Environmental Science & Technology, 44(1):426–431. https://doi.org/10.1021/es9021206
Li XQ, Zhou JS, Zhou QX, et al., 2018. Removal of elemental mercury using titania sorbents loaded with cobalt ceria oxides from syngas. New Journal of Chemistry, 42(15): 12503–12510. https://doi.org/10.1039/C8NJ02069H
Liu YX, Adewuyi YG, 2016. A review on removal of elemental mercury from flue gas using advanced oxidation process: chemistry and process. Chemical Engineering Research and Design, 112:199–250. https://doi.org/10.1016/j.cherd.2016.06.024
Lu DY, Granatstein DL, Rose DJ, 2004. Study of mercury speciation from simulated coal gasification. Industrial & Engineering Chemistry Research, 43(17):5400–5404. https://doi.org/10.1021/ie034121u
Lu H, Greenwood P, Chen TS, et al., 2012. The separate production of H2S from the thermal reaction of hydrocarbons with magnesium sulfate and sulfur: implications for thermal sulfate reduction. Applied Geochemistry, 27(1): 96–105. https://doi.org/10.1016/j.apgeochem.2011.09.007
Matsumoto S, 2004. Recent advances in automobile exhaust catalysts. Catalysis Today, 90(3–4):183–190. https://doi.org/10.1016/j.cattod.2004.04.048
Mullins DR, Overbury SH, Huntley DR, 1998. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surface Science, 409(2):307–319. https://doi.org/10.1016/S0039-6028(98)00257-X
Qi GS, Yang RT, 2004. Characterization and FTIR studies of MnOx-CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. The Journal of Physical Chemistry B, 108(40):15738–15747. https://doi.org/10.1021/jp048431h
Rallo M, Fuente-Cuesta A, Lopez-Anton MA, et al., 2014. Speciation of Hg retained in gasification biomass chars by temperature-programmed decomposition. Fuel Processing Technology, 126:1–4. https://doi.org/10.1016/j.fuproc.2014.04.010
Reddy BM, Khan A, 2005. Nanosized CeO2-SiO2, CeO2-TiO2, and CeO2-ZrO2 mixed oxides: influence of supporting oxide on thermal stability and oxygen storage properties of ceria. Catalysis Surveys from Asia, 9(3):155–171. https://doi.org/10.1007/s10563-005-7552-1
Reddy BM, Khan A, Yamada Y, et al., 2003. Structural characterization of CeO2-TiO2 and V2O5/CeO2-TiO2 catalysts by Raman and XPS techniques. The Journal of Physical Chemistry B, 107(22):5162–5167. https://doi.org/10.1021/jp0344601
Rumayor M, Fernandez-Miranda N, Lopez-Anton MA, et al., 2015. Application of mercury temperature programmed desorption (HgTPD) to ascertain mercury/char interactions. Fuel Processing Technology, 132:9–14. https://doi.org/10.1016/j.fuproc.2014.12.032
Shen BX, Ma HQ, Yao Y, 2012. Mn-CeOx/Ti-PILCs for selective catalytic reduction of NO with NH3 at low temperature. Journal of Environmental Sciences, 24(3): 499–506. https://doi.org/10.1016/S1001-0742(11)60756-0
Wang FC, Yu GS, Gong X, et al., 2009. Research and development of large-scale coal gasification technology. Chemical Industry and Engineering Progress, 28(2):173–180. https://doi.org/10.3321/j.issn:1000-6613.2009.02.001
Wu SJ, Oya N, Ozaki M, et al., 2007. Development of iron oxide sorbents for Hg0 removal from coal derived fuel gas: sulfidation characteristics of iron oxide sorbents and activity for COS formation during Hg0 removal. Fuel, 86(17–18):2857–2863. https://doi.org/10.1016/j.fuel.2007.03.004
Wu X, Duan YF, Yao T, et al., 2019. Mercury removal performance and SO2 resistance of Ce-Mn/TiO2 sorbent. China Environmental Science, 39(6):2336–2343. https://doi.org/10.19674/j.cnki.issn1000-6923.2019.0278
Yang ZQ, Li HL, Liao C, et al., 2018. Magnetic rattle-type Fe3O4@CuS nanoparticles as recyclable sorbents for mercury capture from coal combustion flue gas. ACS Applied Nano Materials, 1(9):4726–4736. https://doi.org/10.1021/acsanm.8b00948
You SL, Zhou JS, Hou WH, et al., 2014. Factors influencing the removal of elemental mercury by Mn-AC sorbent in syngas. Journal of Fuel Chemistry and Technology, 42(11):1324–1331. https://doi.org/10.3969/j.issn.0253-2409.2014.11.008
Yu XQ, Bao JJ, Jiang XX, et al., 2015. Performance and mechanism of catalytic oxidation for mercury by Mn-doped TiO2 catalysts in flue gas. Proceedings of the CSEE, 35(13):3331–3337. https://doi.org/10.13334/j.0258-8013.pcsee.2015.13.016
Yuan B, Mao XZ, Wang Z, et al., 2020. Radical-induced oxidation removal of multi-air-pollutant: a critical review. Journal of Hazardous Materials, 383:121162. https://doi.org/10.1016/j.jhazmat.2019.121162
Zeng XB, Xu Y, Zhang B, et al., 2017. Elemental mercury adsorption and regeneration performance of sorbents FeMnOx enhanced via non-thermal plasma. Chemical Engineering Journal, 309:503–512. https://doi.org/10.1016/j.cej.2016.10.047
Zeng Y, Zhang S, Groves FR, et al., 1999. High temperature gas desulfurization with elemental sulfur production. Chemical Engineering Science, 54(15–16):3007–3017. https://doi.org/10.1016/S0009-2509(98)00427-8
Zhang AC, Zheng WW, Song J, et al., 2014. Cobalt manganese oxides modified titania catalysts for oxidation of elemental mercury at low flue gas temperature. Chemical Engineering Journal, 236:29–38. https://doi.org/10.1016/j.cej.2013.09.060
Zhang H, Zhao JT, Fang YT, et al., 2012. Catalytic oxidation and stabilized adsorption of elemental mercury from coal-derived fuel gas. Energy & Fuels, 26(3):1629–1637. https://doi.org/10.1021/ef201453d
Zhang HW, Chen JY, Zhao K, et al., 2016. Removal of vapor-phase elemental mercury from simulated syngas using semi-coke modified by Mn/Ce doping. Journal of Fuel Chemistry and Technology, 44(4):394–400. https://doi.org/10.1016/S1872-5813(16)30020-2
Zhang SB, Zhao YC, Díaz-Somoano M, et al., 2018. Synergistic mercury removal over the CeMnO3 perovskite structure oxide as a selective catalytic reduction catalyst from coal combustion flue gas. Energy & Fuels, 32(11): 11785–11795. https://doi.org/10.1021/acs.energyfuels.8b02518
Zheng JM, Zhou JS, Luo ZY, et al., 2012. Impact of individual acid flue gas components on mercury capture by heat-treated activated carbon. Journal of Zhejiang University-SCIENCE A (Applied Physics & Engineering), 13(9):700–708. https://doi.org/10.1631/jzus.A1200112
Zhou JS, Hou WH, Qi P, et al., 2013. CeO2-TiO2 sorbents for the removal of elemental mercury from syngas. Environmental Science & Technology, 47(17):10056–10062. https://doi.org/10.1021/es401681y
Zhu YC, Han XJ, Huang ZG, et al., 2018. Superior activity of CeO2 modified V2O5/AC catalyst for mercury removal at low temperature. Chemical Engineering Journal, 337: 741–749. https://doi.org/10.1016/jxej.2017.10.115
Zhuang K, Qiu J, Xu BL, et al., 2012. Promotional effect of cerium oxide on the catalytic properties of Ce-Mn-Ti-O catalysts for selective catalytic reduction of NO. Acta Physico-Chimica Sinica, 28(3):681–685. https://doi.org/10.3866/PKU.WHXB201111141
Author information
Authors and Affiliations
Contributions
Hui CAO wrote the manuscript. Jin-song ZHOU and Qi-xin ZHOU provided the suggestions. Qi-xin ZHOU, Xin-yu XU, and Cong XIE helped to organize the manuscript.
Corresponding author
Additional information
Conflict of interest
Hui CAO, Jin-song ZHOU, Qi-xin ZHOU, Xin-yu XU, and Cong XIE declare that they have no conflict of interest.
Project supported by the National Natural Science Foundation of China (No. 51576173)
Rights and permissions
About this article
Cite this article
Cao, H., Zhou, Js., Zhou, Qx. et al. Elemental mercury removal from coal gas by CeMnTi sorbents and their regeneration performance. J. Zhejiang Univ. Sci. A 22, 222–234 (2021). https://doi.org/10.1631/jzus.A2000079
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.A2000079