Abstract
Objective
The aim of this study was to formulate polymer-based artesunate nanoparticles for malaria treatment.
Methods
Artesunate was loaded with poly(D,L-lactic-co-glycolic acid) (PLGA) by solvent evaporation from an oil-in-water single emulsion. Nanoparticles were characterized by X-ray diffraction and differential scanning calorimetry analyses. In vivo antimalarial studies at 4 mg/kg were performed on Swiss male albino mice infected with Plasmodium berghei. Hematological and hepatic toxicity assays were performed. In vitro cytotoxicity of free and encapsulated artesunate (Art-PLGA) to cell line RAW 264.7 was determined at concentrations of 7.8–1000 μg/ml.
Results
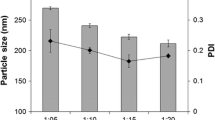
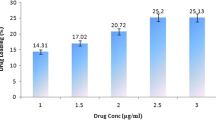
The particle size of the formulated drug was (329.3±21.7) nm and the entrapment efficiency was (38.4±10.1)%. Art-PLGA nanoparticles showed higher parasite suppression (62.6%) compared to free artesunate (58.2%, P<0.05). Platelet counts were significantly higher in controls (305 000.00±148 492.40) than in mice treated with free artesunate (139 500.00±20 506.10) or Art-PLGA (163 500.00±3535.53) (P<0.05). There was no sign of hepatic toxicity following use of the tested drugs. The half maximal inhibitory concentration (IC50) of Art-PLGA (468.0 μg/ml) was significantly higher (P<0.05) than that of free artesunate (7.3 μg/ml) in the in vitro cytotoxicity assay.
Conclusions
A simple treatment of PLGA-entrapped artesunate nanoparticles with dual advantages of low toxicity and better antiplasmodial efficacy has been developed.
概要
目 的
为疟疾治疗制定基于聚合物的青蒿琥酯纳米粒。
创新点
以聚乳酸羟乙酸共聚物 (PLGA) 为载体, 制备青蒿琥酯纳米颗粒。 并以小鼠为模型, 评估其抗 疟疗效和安全性。
方 法
以 PLGA 为载体, 采用从单一的水包油乳剂中进行溶剂蒸发的方法制备青蒿琥酯纳米颗粒。 借助 X 射线衍射和差示扫描量热分析对纳米颗粒进行表征。 以 4 mg/kg 的剂量对感染疟原虫的雄性瑞士白化小鼠进行体内抗疟活性的研究, 测定血液和肝毒性的相关指标。 体外实验以小鼠腹腔巨噬细胞细胞系 RAW 264.7 为模型, 在 7.8∼1000 μg/ml 浓度范围内, 测定游离型和包裹型青蒿琥酯的细胞毒性。
结 论
实验结果表明, 纳米颗粒的粒径为 (329.3± 21.7) nm, 包封率为(38.4±10.1)%。 与游离青蒿琥酯 (58.2%) 相比, 基于 PLGA 的青蒿琥酯纳米颗粒 (Art-PLGA) 具有较高的抑虫率 (62.6%), P<0.05 。 就血小板计数结果而言, 对照组 (305 000.00±148 492.40) 明显地高于游离青蒿琥酯组 (139 500.00±20 506.10) 和 Art-PLGA 组 (163 500.00±3535.53), P<0.05。 因此, 药物的使用没有导致肝毒性的产生。 体外细胞毒性试验结果表明, Art-PLGA 的半数抑制浓度 (IC50, 468.0 μg/ml) 显著高于游离青蒿琥酯 (7.3 μg/ml), P<0.05。 基于 PLGA 的青蒿琥酯纳米颗粒是一种有效安全的抗疟治疗方法。
Similar content being viewed by others
References
Acharya, S., Sahoo, S.K., 2011. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Del. Rev., 63(3):170–183. http://dx.doi.org/10.1016/j.addr.2010.10.008
Agnihotri, J., Singh, S., Bigonia, P., 2013. Formal chemical stability analysis and solubility analysis of artesunate and hydroxychloroquinine for development of parenteral dosage form. J. Pharm. Res., 6:117-122. http://dx.doi.org/10.1016/j.jopr.2012.11.025
Anitha, A., Deepagan, V.G., Rani, V.V.D., et al., 2011. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohyd. Poly., 84(3):1158–1164. http://dx.doi.org/10.1016/j.carbpol.2011.01.005
Bhawana, R.K., Basniwal, H.S., Buttal, V.K., et al., 2011. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem., 59(5): 2056–2061. http://dx.doi.org/10.1021/jf104402t
Bigoniya, P., Sahu, T., Tiwari, V., 2015. Hematological and biochemical effects of sub-chronic artesunate exposure in rats. Toxicol. Rep., 2:280-288. http://dx.doi.org/10.1016/j.toxrep.2015.01.007
Chadha, R., Gupta, S., Pathak, N., 2012. Artesunate-loaded chitosan/lecithin nanoparticles: preparation, characterization, and in vivo studies. Drug Dev. Ind. Pharm., 38(12):1538–1546. http://dx.doi.org/10.3109/03639045.2012.658812
Cheesbrough, M., 1998. District Laboratory Practice in Tropical Countries. Part 1. Cambridge University Press, London.
Chinaeke, E.E., Chime, S.A., Onyishi, V.I., et al., 2015. Formulation development and evaluation of the anti-malaria properties of sustained release artesunate-loaded solid lipid microparticles based on phytolipids. Drug Deliv., 22(5):652–665. http://dx.doi.org/10.3109/10717544.2014.881633
Chittasupho, C., Xie, S.X., Baoum, A., et al., 2009. ICAM-1 targeting of doxorubicinloaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci., 37(2):141–150. http://dx.doi.org/10.1016/j.ejps.2009.02.008
Clark, R.L., 2012. Effects of artemisinins on reticulocyte counts and and relationship to possible embryotoxicity in confirmed and unconfirmed malarial patients. Birth Def. Res. Part A: Clin. Mol. Teratol., 94(2):61–75. http://dx.doi.org/10.1002/bdra.22868
Cooper, D.L., Harirforoosh, S., 2014. Design and optimization of PLGA-based diclofenac loaded nanoparticles. PLoS ONE, 9(1):e87326. http://dx.doi.org/10.1371/journal.pone.0087326
des Rieux, A., Fievez, V., Garinot, M., et al., 2006. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control Release, 116(1):1–27. http://dx.doi.org/10.1016/j.jconrel.2006.08.013
Dikasso, D., Makonnen, E., Debella, A., et al., 2006. In vivo anti-malaria activity of hydroalcoholic extract from Asparaginus africanus in mice infected with Plasmodium berghei. Ethiop. J. Health Dev., 20(2):112–118. http://dx.doi.org/10.4314/ejhd.v20i2.10021
Efferth, T., 2007. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin-from bench to bedside. Planta Med., 73(4):299–309. http://dx.doi.org/10.1055/s-2007-967138
Faber, J.L., Chein, K.R., Mitlnacht, S., 1981. Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am. J. Pathol., 102(2):271–281.
Ibrahim, N., Ibrahim, H., Dormoi, J., et al., 2014. Albuminbound nanoparticles of practically water-soluble antimalarial lead greatly enhance its efficacy. Int. J. Pharm., 464(1–2):214–224. http://dx.doi.org/10.1016/j.ijpharm.2014.01.001
Jain, R.A., 2000. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials, 21(23):2475–2490. http://dx.doi.org/10.1016/S0142-9612(00)00115-0
Kumari, A., Yadav, S.K., Yadav, S.C., 2010. Biodegradable polymeric nanoparticles based drug delivery systems. Coll. Surf. B: Biointer., 75(1):1–18. http://dx.doi.org/10.1016/j.colsurfb.2009.09.001
Lai, S.K., O’ Hanlon, D.E., Harrold, S., et al., 2007. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA, 104(5): 1482–1487. http://dx.doi.org/10.1073/pnas.0608611104
Liu, W.M., Gravett, A.M., Dalgleish, A.G., 2011. The antimalarial agent artesunate possesses anticancer properties that can be enhanced by combination strategies. Int. J. Cancer, 128(6):1471–1480. http://dx.doi.org/10.1002/ijc.25707
Mainardes, R.M., Evangelista, R.C., 2005. PLGA nanoparticles containing praziquantel effect of formulation variables on size distribution. Int. J. Pharm., 290(1–2):137–144. http://dx.doi.org/10.1016/j.ijpharm.2004.11.027
McNeil, S.E., 2005. Nanotechnology for the biologist. J. Leuk. Biol., 78(3):585–592. http://dx.doi.org/10.1189/jlb.0205074
Meng, H., Xu, K., Xu, Y., et al., 2014. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Coll. Surf. B: Biointer., 115:164-169. http://dx.doi.org/10.1016/j.colsurfb.2013.11.039
Mesembe, O.E., Ivang, A.E., Udo-Attah, G., et al., 2004. A morphometric study of the teratogenic effect of artesunate on the central nervous system of the Wistar rats foetus. Nig. J. Physiol. Sci., 19(1):92–97.
National Research Council, 2010. Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, DC.
Nguyen, H.T., Tran, T.H., Kim, J.O., et al., 2015. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly D,L-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res., 38(5):716–724. http://dx.doi.org/10.1007/s12272-014-0424-3
Nobbmann, U.L.F., 2014. Polydispersity—what does it mean for DLS and chromatography? http://www.materials-talks.com/blog/2014/10/23/polydispersity-what-does-it-meanfor-dls-and-chromatography [accessed on Oct. 24, 2016]
Nordström, P., 2011. Formulation of Polymeric Nanoparticles Encapsulating and Releasing a New Hydrophobic Cancer Drug. MS Thesis, Chalmers University of Technology, Göteborg, Sweden.
Panda, A., Meena, J., Katara, R., et al., 2016. Formulation and characterization of clozapine and risperidone co-entrapped spray-dried PLGA nanoparticles. Pharm. Dev. Technol., 21(1):43–53. http://dx.doi.org/10.3109/10837450.2014.965324
Pradhan, R., Poudel, B.K., Ramasamy, T., et al., 2013. Docetaxel loaded polylactic acid-co-glycolic acid nanoparticles: formulation, physicochemical characterization and cytotoxicity studies. J. Nanosci. Nanotechnol., 13(8): 5948–5956. http://dx.doi.org/10.1166/jnn.2013.7735
Tona, L., Mesia, K., Ngimbi, N.P., et al., 2001. In vivo antimalarial activity of Cassia occindentalis, Morinda morindoides and Phyllanthus niruri. Ann. Trop. Med. Parasitol., 95(1):47–57. http://dx.doi.org/10.1080/00034983.2001.11813614
White, T.E., Bushdid, P.B., Ritter, S., et al., 2006. Artesunate-induced depletion of embryonic erythroblasts precedes embryolethality and teratogenicity in vivo. Birth Def. Res. Part B: Dev. Reprod. Toxicol., 77(5):413–429. http://dx.doi.org/10.1002/bdrb.20092
Woerdenbag, H.J., Moskal, T.A., Pras, N., et al., 1993. Cytotoxicity of artemisinin-related endoperoxides to Ehrlich ascites tumor cells. J. Nat. Prod., 56(6):849–856. http://dx.doi.org/10.1021/np50096a007
Wohlfart, S., Khalansky, A.S., Gelperina, S., et al., 2011. Efficient chemotherapy of rat glioblastoma using doxorubicinloaded PLGA nanoparticles with different stabilizers. PLoS ONE, 6: e19121. http://dx.doi.org/10.1371/journal.pone.0019121
Zhang, Z.P., Feng, S.S., 2006. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials, 27(21):4025–4033. http://dx.doi.org/10.1016/j.biomaterials.2006.03.006
Acknowledgements
Oyetunde OYEYEMI acknowledges the Centre for Science and Technology of the Non-Aligned and Other Developing Countries (NAM S&T Centre) in collaboration with the Department of Science & Technology (DST), the Government of India for “Research Training Fellowship for Developing Country Scientists (RTF-DCS)” award, undertaken at the National Institute of Immunology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dauda, K., Busari, Z., Morenikeji, O. et al. Poly(D,L-lactic-co-glycolic acid)-based artesunate nanoparticles: formulation, antimalarial and toxicity assessments. J. Zhejiang Univ. Sci. B 18, 977–985 (2017). https://doi.org/10.1631/jzus.B1600389
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1600389