Abstract
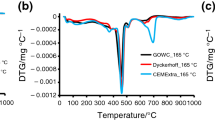
The present study investigates the thermal evolution of hydrates in carbonation-cured Portland cement. Paste samples were placed in a carbonation chamber after the 24 h of initial curing, while reference samples were cured in a sealed condition until 28 days. Thermogravimetry, unconfined strength tests, X-ray diffractometry, and solid-state 29Si and 27Al MAS NMR spectroscopy were conducted. The results showed that the binder gel in carbonation-cured cement shares some structural similarities with aluminosilicate glass in terms of Si and Al analogues. This characteristic was also reflected by its thermogravimetric behavior, presenting much less weight loss associated with dehydration in comparison with hydrated cement. However, the binder gel in carbonation-cured cement underwent depolymerization into monomeric Si at 800 °C, similar to hydrated cement. Moreover, carbonation-cured cement underwent crystallization pathway identical to that of hydrated cement, displaying a hydrate-like thermal behavior.






Similar content being viewed by others
References
Jang JG, Kim GM, Kim HJ, Lee HK (2016) Review on recent advances in CO2 utilization and sequestration technologies in cement-based materials. Constr Build Mater 127:762–773
Branch JL, Kosson DS, Garrabrants AC, He PJ (2016) The impact of carbonation on the microstructure and solubility of major constituents in microconcrete materials with varying alkalinities due to fly ash replacement of ordinary Portland cement. Cem Concr Res 89:297–309
Morrison J, Jauffret G, Galvez-Martos JL, Glasser FP (2016) Magnesium-based cements for CO2 capture and utilisation. Cem Concr Res 85:183–191
Juenger MCG, Siddique R (2015) Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem Concr Res 78:71–80
Gartner E, Hirao H (2015) A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem Concr Res 78:126–142
Bernal SA, Juenger MCG, Ke X, Matthes W, Lothenbach B, De Belie N, Provis JL (2017) Characterization of supplementary cementitious materials by thermal analysis. Mater Struct 50:26
Juenger MCG, Winnefeld F, Provis JL, Ideker JH (2011) Advances in alternative cementitious binders. Cem Concr Res 41:1232–1243
Lothenbach B, Scrivener K, Hooton RD (2011) Supplementary cementitious materials. Cem Concr Res 41:1244–1256
Xi F, Davis SJ, Ciais P, Crawford-Brown D, Guan D, Pade C, Shi T, Syddall M, Lv J, Ji L, Bing L (2016) Substantial global carbon uptake by cement carbonation. Nat Geosci 9:880–883
Jang JG, Lee HK (2016) Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem Concr Res 82:50–57
Bertos MF, Simons SJR, Hills CD, Carey PJ (2004) A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J Hazard Mater 112:193–205
Rostami V, Shao Y, Boyd A (2012) Carbonation curing versus steam curing for precast concrete production. J Mater Civ Eng 24:1221–1229
Fang Y, Chang J (2017) Rapid hardening β-C2S mineral and microstructure changes activated by accelerated carbonation curing. J Therm Anal Calorim 129:681–689
Chang J, Fang Y, Shang X (2016) The role of β-C2S and γ-C2S in carbon capture and strength development. Mater Struct 49:4417–4424
Shtepenko O, Hills C, Brough A, Thomas M (2006) The effect of carbon dioxide on β-dicalcium silicate and Portland cement. Chem Eng J 118:107–118
Goto S, Suenaga K, Kado T (1995) Calcium silicate carbonation products. J Am Ceram Soc 78:2867–2872
Goodbrake CJ, Young JK, Berger RL (1979) Reaction of γ-C2S and C3S with CO2 and water vapor. J Am Ceram Soc 62:168–171
Morandeau AE, White CE (2015) In situ X-ray pair distribution function analysis of accelerated carbonation of a synthetic calcium-silicate-hydrate gel. J Mater Chem A 3:8597–8605
Sevelsted TF, Skibsted J (2015) Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem Concr Res 71:56–65
Suzuki K, Nishikawa T, Ito S (1985) Formation and carbonation of C–S–H in water. Cem Concr Res 15:213–224
Kutchko BG, Strazisar BR, Lowry GV, Dzombak DA, Thaulow N (2007) Degradation of well cement by CO2 under geologic sequestration conditions. Environ Sci Technol 41:4787–4792
Kutchko BG, Strazisar BR, Lowry GV, Dzombak DA, Thaulow N (2008) Rate of CO2 attack on hydrated Class H well cement under geologic sequestration conditions. Environ Sci Technol 42:6237–6242
Park SM, Jang JG, Lee NK, Lee HK (2016) Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem Concr Res 89:72–79
Kim GM, Jang JG, Naeem F, Lee HK (2015) Heavy metal leaching, CO2 uptake and mechanical characteristics of carbonated porous concrete with alkali-activated slag and bottom ash. Int J Concr Struct Mater 9:283–294
Jeong Y, Park H, Jun Y, Jeong JH, Oh JE (2016) Influence of slag characteristics on strength development and reaction products in a CaO-activated slag system. Cem Concr Compos 72:155–1673
Jang JG, Kim HJ, Park SM, Lee HK (2015) The influence of sodium hydrogen carbonate on the hydration of cement. Constr Build Mater 94:746–749
Park H, Jeong Y, Jun Y, Jeong JH, Oh JE (2016) Strength enhancement and pore-size refinement in clinker-free CaO-activated GGBFS systems through substitution with gypsum. Cem Concr Compos 68:57–65
Alonso C, Fernandez L (2004) Dehydration and rehydration processes of cement paste exposed to high temperature environments. J Mater Sci 39:3015–3024
Andersen MD, Jakobsen HJ, Skibsted J (2006) A new aluminum-hydrate species in hydrated Portland cements characterized by 27Al and 29Si MAS NMR spectroscopy. Cem Concr Res 36:3–17
Nishikawa T, Suzuki K, Ito S (1992) Decomposition of synthesized ettringite by carbonation. Cem Concr Res 22:6–14
Acknowledgements
This study was supported by the Saudi Aramco-KAIST CO2 Management Center to whom the authors are grateful. The authors acknowledge the use of solid state NMR spectrometer at Korea Basic Science Institute Western Seoul center, and would like to thank Dr. Seen-Ae Chae for assistance with NMR spectroscopy.
Funding
The participation of S.M. Park, J.H. Seo and H.K. Lee was funded by the Saudi Aramco-KAIST CO2 Management Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, S.M., Seo, J.H. & Lee, H.K. Thermal evolution of hydrates in carbonation-cured Portland cement. Mater Struct 51, 7 (2018). https://doi.org/10.1617/s11527-017-1114-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-017-1114-7