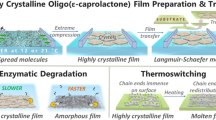
Abstract
The potential of using crystallinity as morphological parameter to control polyester degradation in acidic environments is explored in ultrathin films by Langmuir technique. Films of hydroxy or methacrylate end-capped oligo(ε-caprolactone) (OCL) are prepared at the air–water interface as a function of mean molecular area (MMA). The obtained amorphous, partially crystalline or highly crystalline ultrathin films of OCL are hydrolytically degraded at pH ~ 1.2 on water surface or on silicon surface as-transferred films. A high crystallinity reduces the hydrolytic degradation rate of the films on both water and solid surfaces. Different acceleration rates of hydrolytic degradation of semi-crystalline films are achieved either by crystals complete melting, partially melting, or by heating them below their melting temperatures. Semi-crystalline OCL films transferred via water onto a solid surface retain their crystalline morphology, degrade in a controlled manner, and are of interest as thermoswitchable coatings for cell substrates and medical devices.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the primary benefits of choosing poly(ε-caprolactone) (PCL) as material for drug release systems and medical implants is its (bio)degradability. Besides the hydrolysis rate of the ester bonds, the degradation behavior of PCL is also influenced by its crystallinity, since the tight assembly of polymer chains restricts the access of water/ions to cleavable chemical bonds [1]. Another feature making PCL a promising candidate material for medical devices is that its crystals can melt around the body temperature, thereby enabling shape-shifting of implants [2]. Since the overall degradation profile of PCL devices depends on their crystalline morphology, implementing or executing a shape-shifting capability affects their degradation behavior [3].
It is therefore highly relevant to characterize how crystallinity and activation of thermal transitions affect the degradation behavior of PCL, yet this is difficult in bulk systems.
Degradation studies of bulk PCL in pellets, scaffolds, fibers, or thick films can take weeks to months. At pH 1, bulk PCL undergoes an induction period (~ 300 h) prior to exhibiting steady degradation [4]. To shorten the degradation time, frequently studies employ extreme pH values or high temperatures [5]. Care has to be taken when extrapolating their results to physiological conditions [6]. For extreme pH values, the bond scission rate is accelerated, while the water diffusion is not, which favors surface erosion [5]. Also, catalytic effects might be so strong that crystalline and amorphous bonds are broken equally, while elevated temperatures result in the melting of crystals. Moreover, it is extremely challenging to determine the degradation rates of crystalline domains, since degradation can induce chemi-crystallization, resulting in an interplay between degradation and formation of crystallites [7].
Therefore, here we use ultrathin films of semi-crystalline PCL as model systems for systematic and fast evaluation of the influence of crystals on the degradation profile of PCL.
By means of the Langmuir technique, we are able to generate ultrathin OCL films at the air–liquid interface with precisely adjusted semi-crystalline morphology, including crystal size, number density, thickness, and melting temperature. The polymer films with adjustable semi-crystalline morphology are transferable to solid substrates for further characterization [8]. It is also possible to subject these films to thermal treatments that reflect the activation of a shape-shifting effect while measuring the influence on degradation in real time. We therefore hypothesize that the degradation rate of chain segments in amorphous and crystalline domains can be determined systematically in ultrathin films prepared using different OCL telechelics.
In this study, semi-crystalline or amorphous films of hydroxy (OCL diol; OCDOL) and methacrylate (OCL dimethacrylate; OCDME) end-capped linear oligomers are prepared at the air–water interface at pH 7 at 21 °C (Fig. 1a). The prepared films are hydrolytically degraded by shifting the pH to ~ 1.2. Amorphous films are degraded at different surface pressures to prove an influence of OCL chain-packing on the hydrolytic degradation (Fig. 1b:1). Partially crystalline OCL films are hydrolytically degraded at different constant degradation temperatures (Tdeg) close to the melting and recrystallization range (Fig. 1b:2). These are compared to highly crystalline films, where the influence of OCL end groups (hydroxy or methacrylate) or temperature (21 or 37 °C) on degradation is determined (Fig. 1b:3). Both highly crystalline and molten films are transferred to silicon surfaces to further investigate their change in morphology during degradation. (Fig. 1b:3).
Experimental
Materials
Oligo(ε-caprolactone) diol (OCDOL, trade name CAPA 2803, Solvay Caprolactones, Warrington, U.K.) and oligo(ε-caprolactone) dimethacrylate (OCDME, Sigma-Aldrich, Germany) were used without any further purification. Bulk material characteristics of OCDOL and OCDME are summarized in Table S1 of supplementary information.
Preparation of OCL ultrathin films at air–water interface by Langmuir technique
Langmuir technique setup and details on the cleaning of the Teflon trough used for investigation of OCL films at air–water interface were described previously [8]. Briefly, to prepare OCL ultrathin films differing in their crystallinity, chloroform solution (0.2—0.3 mg mL−1) of OCDOL or OCDME was spread dropwise on the surface of water at 21 ± 0.5 °C in the Langmuir trough using a micro syringe (Hamilton Co., Reno, NV, USA). After a waiting time of 30 min for chloroform evaporation, the film was symmetrically compressed to specific MMA (see supplementary information; Table S2) at a barrier speed of 10 mm min−1. MMA is the mean surface area occupied per ε‐caprolactone repeat unit at the air–water interface and is calculated from the number of spread repeat units and the total area of the film. Details of hydrolytic degradation of OCL films at the air–water interface or of Langmuir-Schaefer (LS) transferred films at silicon surface are described in supplementary information; Table S3.
Statistics experimental errors and error considerations with respect to instrumentation
OCL films preparation and hydrolytic degradation at the air–water interface or of LS transferred films at silicon surface were performed at least three times. The systematic error range of the surface pressure sensor is ± 0.7 mN m−1 and ± 0.2 °C for the temperature sensor. At the air–water interface, the experimental error during spreading of polymer solution and environmental impurities like dust/lint particles can lead to irregularities in OCL film MMA measurements by ± 1 Å2.
Results and discussion
Ultrathin films of OCDOL or OCDME were prepared at the air–water interface by compressing molecules to different MMAs (~ 42 or 37 Å2 = amorphous films; 8.5 Å2 = partially crystalline; ~ 2 Å2 = highly crystalline) using the Langmuir trough barriers. The related compression isotherms (MMA versus surface pressure) and morphology of OCL ultrathin films on water at pH 7 under room temperature are shown in supplementary information Fig. S1. A detailed explanation on differences in the compression isotherms, crystallization, and melting behavior of OCDOL and OCDME films at the air–water interface can be found elsewhere [8,9,10].
Figure 2a shows the hydrolytic degradation of amorphous OCL films at two different surface pressures, where 6.5 mN m−1 is closer to the crystallization transition than 5 mN m−1 [9]. During a 24-h period at pH 7, amorphous OCL films at 6.5 mN m−1 (Fig. 2a) show an area reduction of about 5%. This confirms that the film is stable enough to carry out long-term degradation studies at both surface pressures. The degradation curve of amorphous OCLs at 6.5 mN m−1 shows the typical sigmoid shape of solubility-controlled polymer degradation (Fig. 2a). Since the molecular weight is initially far above the solubility threshold, chain cuts have a low probability of producing short, water-soluble fragments. Once the molecular weight is sufficiently low, chain cuts have a high probability of creating water-soluble fragments. The slightly faster dissolution of OCDME compared to OCDOL is expected due to its lower initial molecular mass. At a lower surface pressure of 5 mN m−1, the OCL films are even more stable at pH 7 (Fig. 2a). When changing the pH to 1.2, an almost linear mass loss is observed, resulting in slower erosion than at higher surface pressure (Fig. 2a). Such a pressure dependence of the degradation curve could be explained by the surface-pressure-dependent solubility of fragments. In general, the higher the surface pressure, the greater the tendency for molecules to desorb from the interface. That means that at lower surface pressure, the chance that small fragments remain at the surface is higher. Then, the solubility transition becomes less sharp and the effect of initial molecular weight becomes even smaller (Fig. 2a).
The hydrolytic degradation of partially crystalline films (crystallized at MMA 8.5 Å2; 21 °C) on water at pH 1.2 at different temperatures is shown in supplementary information; Fig. S2. Partially crystalline films display competition of melting and degradation, together with the abundance of much faster degrading amorphous segments, and makes them a far from ideal system to study the influence of crystallinity on OCL degradation.
To get a clear understanding of the degradation rate of OCL crystals, highly crystalline OCDOL and OCDME films were degraded at 21 °C (room temperature) or 37 °C (body temperature) at the air–water interface (Fig. 2b). At 21 °C, pH 1.2 highly crystalline films (Fig. 2b) are far more stable against hydrolytic degradation than partially crystalline films (supplementary information; Fig. S2) or amorphous films (Fig. 2a). The deceleration is on the order of three times for OCDME, and even greater for OCDOL, where no dissolution was observed. Heating the highly crystalline films to body temperature increased the degradation rate (Fig. 2b). For OCDME, the effect was again well within the range expected for thermal activation, i.e., it does not seem that there are additional accelerating effects like partial crystal melting (the highly crystalline films have a much higher Tm (47 to 49 °C) than the partially crystalline ones (27 to 43 °C)). For OCDOL, the effect cannot be quantified since no degradation was observed of its highly crystalline films at room temperature at the air–water interface (Fig. 2b).
Highly crystalline OCDME films at 21 or 37 °C degrade faster than highly crystalline OCDOL films at the air–water interface (Fig. 2b). This effect is attributed to differences in chain-packing and larger free volume in OCDME crystals, which is evidenced by the prevalence of growth sectors with a lower melting temperature [8]. Nevertheless, for both OCDOL and OCDME, the highly crystalline films degrade slowly and almost linearly at 37 °C. Such a behavior is highly demanded for applications like erosion-controlled drug release, but very different from the mechanism observed for erosion of highly crystalline OCL layers under enzymatic catalysis [10], where the sigmoid-shaped mass loss indicated a solubility-controlled degradation.
To gain further insights into the difference between degradation of amorphous and crystalline OCL under acidic conditions, highly crystalline OCL films and amorphous films (melted highly crystalline films at 49 °C) were transferred to silicon substrates by the LS method (Fig. 3). Where OCL crystals are transferable with minor fractures, the amorphous films show dewetting (Fig. 3). All LS transferred films were hydrolytically degraded by incubation under water at pH 1.2 at 37 °C (Fig. 3).
Degradation of transferred highly crystalline and amorphous (melted) films of OCDOL and OCDME transferred on silicon at 37 °C; pH 1.2 resulted in visible differences in the crystal morphologies after 72 h (Fig. 3). It is very clear that under these conditions, the crystals are not shrinking laterally in a homogeneous way. If they were, an exponentially decreasing degradation rate, which is proportional to the remaining area, would be expected. Rather, cracks and channels are generated within the crystals. The existence of regions with different perfection of chain-packing was also concluded from enzymatic degradation experiments [11]. Therefore, the linear degradation of the single crystals is attributed to the propagation of the degradations through these regions of different chain-packing. Compared to the single crystals, the films, which were transferred in a molten state were nearly completely degraded after 72 h (Fig. 3).
Conclusion
Amorphous and semi-crystalline films of dihydroxy- or dimethacrylate-terminated linear OCL were prepared by compression of molecules to different MMAs on water at 21 °C and pH 7. The prepared films were hydrolytically degraded at pH 1.2 at different physiologically applicable temperatures. At room temperature, the mass loss of highly crystalline OCL diol films was negligible for 24 h, proving that crystals are hydrolytically far more stable than amorphous chains. In general, the highly crystalline OCL layers displayed linear mass loss, making them attractive candidates, for e.g., drug-releasing coatings. The strongly retarded degradation of OCL crystals was also observed after transfer to solid substrates. The perfection in chain-packing seems to play an important part in the degradation of OCL crystals, as evidenced by pattern formation and lower hydrolytic stability of crystals with lower melting temperature. An acceleration of degradation was observed upon melting of semi-crystalline layers, but the effect was fully reversible when the layers were cooled back to the initial temperature. The present work demonstrates the option to control the hydrolytic degradation of OCL physically via crystallization and chemically via chain termination. Future investigations could encompass adjusting the melting temperature of highly crystalline layers to produce functional coatings that degrade in response to a defined thermal stimulus.
Data availability
Data will be made available on reasonable request.
References
M. Bartnikowski, T.R. Dargaville, S. Ivanovski, D.W. Hutmacher, Prog. Polym. Sci. 96, 1 (2019)
A. Lendlein, O.E.C. Gould, Nat. Rev. Mater. 4(2), 116 (2019)
R. Machatschek, S. Saretia, A. Lendlein, Adv. Mater. Interfaces 8(6), 2001926 (2021)
J.H. Jung, M. Ree, H. Kim, Catal. Today 115(1), 283 (2006)
L.N. Woodard, M.A. Grunlan, ACS Macro Lett. 7(8), 976 (2018)
H. Sun, L. Mei, C. Song, X. Cui, P. Wang, Biomaterials 27(9), 1735 (2006)
D.C. França, D.D. Morais, E.B. Bezerra, E.M. Araújo, R.M.R. Wellen, Mater. Res. 21, 1–8 (2018)
S. Saretia, R. Machatschek, T. Bhuvanesh, A. Lendlein, Adv. Mater. Interfaces 200, 2021 (1940)
S. Saretia, R. Machatschek, B. Schulz, A. Lendlein, Biomed. Mater. 14(3), 034103 (2019)
S. Saretia, R. Machatschek, A. Lendlein, MRS Adv. (2021). https://doi.org/10.1557/s43580-021-00020-5
T. Iwata, Y. Doi, Polym. Int. 51(10), 852 (2002)
Acknowledgments
The authors acknowledge Dr. Natalia Tarazona and Ms. Manuela Keller for their assistance in Langmuir setup. This work is financially supported by the Helmholtz Association of German Research Centers through program-oriented funding and through Helmholtz Graduate School for Macromolecular Bioscience (MacroBio, VH-GS-503).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saretia, S., Machatschek, R. & Lendlein, A. Degradation kinetics of oligo(ε-caprolactone) ultrathin films: Influence of crystallinity. MRS Advances 6, 790–795 (2021). https://doi.org/10.1557/s43580-021-00067-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43580-021-00067-4