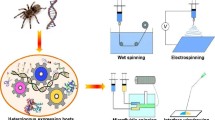
Abstract
The overarching aim of biomimetic approaches to materials synthesis is to mimic simultaneously the structure and function of a natural material, in such a way that these functional properties can be systematically tailored and optimized. In the case of synthetic spider silk fibers, to date functionalities have largely focused on mechanical properties. A rapidly expanding body of literature documents this work, building on the emerging knowledge of structure–function relationships in native spider silks, and the spinning processes used to create them. Here, we describe some of the benchmark achievements reported until now, with a focus on the last five years. Progress in protein synthesis, notably the expression on full-size spidroins, has driven substantial improvements in synthetic spider silk performance. Spinning technology, however, lags behind and is a major limiting factor in biomimetic production. We also discuss applications for synthetic silk that primarily capitalize on its nonmechanical attributes, and that exploit the remarkable range of structures that can be formed from a synthetic silk feedstock.





Similar content being viewed by others
References
W.A. Shear, J.M. Palmer, J.A. Coddington, and P.M. Bonamo: A Devonian spinneret: Early evidence of spiders and silk use. Science 246, 479 (1989).
J.M. Gosline, P.A. Guerette, C.S. Ortlepp, and K.N. Savage: The mechanical design of spider silks: From fibroin sequence to mechanical function. J. Exp. Biol. 202, 3295 (1999).
F. Vollrath and D.P. Knight: Liquid crystalline spinning of spider silk. Nature 410, 541 (2001).
A. Rising: Controlled assembly: A prerequisite for the use of recombinant spider silk in regenerative medicine?Acta Biomater. 10, 1627 (2014).
U. Slotta, N. Mougin, L. Römer, and A.H. Leimer: Synthetic spider silk proteins and threads. Chem. Eng. Prog. 108, 43 (2012).
M. Humenik, A.M. Smith, and T. Scheibel: Recombinant spider silks—Biopolymers with potential for future applications. Polymers 3, 640 (2011).
M. Widhe, J. Johansson, M. Hedhammar, and A. Rising: Current progress and limitations of spider silk for biomedical applications. Biopolymers 97, 468 (2012).
O. Tokareva, M. Jacobsen, M. Buehler, J. Wong, and D.L. Kaplan: Structure–function–property–design interplay in biopolymers: Spider silk. Acta Biomater. 10, 1612 (2014).
O. Tokareva, V.A. Michalczechen-Lacerda, E.L. Rech, and D.L. Kaplan: Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 6, 651 (2013).
M. Widhe, J. Johansson, M. Hedhammar, and A. Rising: Invited review: Current progress and limitations of spider silk for biomedical applications. Biopolymers 97, 468 (2012).
D. Bittencourt, P.F. Oliveira, F. Prosdocimi, and E.L. Rech: Protein families, natural history and biotechnological aspects of spider silk. Genet. Mol. Res. 11, 2360 (2012).
A. Tarakanova and M.J. Buehler: A materiomics approach to spider silk: Protein molecules to webs. JOM 64, 214 (2012).
Y. Yang, X. Chen, Z. Shao, P. Zhou, D. Porter, D.P. Knight, and F. Vollrath: Toughness of spider silk at high and low temperatures. Adv. Mat. 17, 84 (2005).
E.M. Pogozelski, W.L. Becker, B.D. See, and C.M. Kieffer: Mechanical testing of spider silk at cryogenic temperatures. Int. J. Biol. Macromol. 48, 27 (2011).
F. Vollrath and D. Porter: Silks as ancient models for modern polymers. Polymer 50, 5623 (2009).
I. Agnarsson, M. Kuntner, and T.A. Blackledge: Bioprospecting finds the toughest biological material: Extraordinary silk from a giant riverine orb spider. PLoS One 5, 1 (2010).
M. Gregorič, I. Agnarsson, T.A. Blackledge, and M. Kuntner: Darwin’s bark spider: Giant prey in giant orb webs (Caerostris darwini, Araneae: Araneidae)?J. Arachnol. 39, 287 (2011).
I. Agnarsson, C. Boutry, and T.A. Blackledge: Spider silk aging: Initial improvement in a high performance material followed by slow degradation. J. Exp. Zool. A Ecol. Genet. Physiol. 309A, 494 (2008).
B.O. Swanson, T.A. Blackledge, A.P. Summers, and C.Y. Hayashi: Spider dragline silk: Correlated and mosaic evolution in high-performance biological materials. Evolution 60, 2539 (2006).
J. Asrar and J.C. Hill: Biosynthetic processes for linear polymers. J. Appl. Polym. Sci. 83, 457 (2002).
E. Munch, M.E. Launey, D.H. Alsem, E. Saiz, A.P. Tomsia, and R.O. Ritchie: Tough, bio-inspired hybrid materials. Science 322, 1516 (2008).
C.P. Brown, C. Harnagea, H.S. Gill, A.J. Price, E. Traversa, S. Licoccia, and F. Rosei: Rough fibrils provide a toughening mechanism in biological fibers. ACS Nano 6, 1961 (2012).
S. Keten and M.J. Buehler: Geometric confinement governs the rupture strength of H-bond assemblies at a critical length scale. Nano Lett. 8, 743 (2008).
A. Sponner, W. Vater, S. Monajembashi, E. Unger, F. Grosse, and K. Weisshart: Composition and hierarchical organisation of a spider silk. PLoS One 2, e998 (2007).
K.J. Koski, P. Akhenblit, K. McKiernan, and J.L. Yarger: Non-invasive determination of the complete elastic moduli of spider silks. Nat. Mater. 12, 262 (2013).
M. Xu and R.V. Lewis: Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. U. S. A. 87, 7120 (1990).
M. Heim, L. Romer, and T. Scheibel: Hierarchical structures made of proteins. The complex architecture of spider webs and their constituent silk proteins. Chem. Soc. Rev. 39, 156 (2010).
A.H. Simmons, C.A. Michal, and L.W. Jelinski: Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 271, 84 (1996).
B. Bonev, S. Grieve, M.E. Herberstein, A.I. Kishore, A. Watts, and F. Separovic: Orientational order of Australian spider silks as determined by solid-state NMR. Biopolymers 82, 134 (2006).
J.D. van Beek, S. Hess, F. Vollrath, and B.H. Meier: The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. U. S. A. 99, 10266 (2002).
F. Vollrath and D. Porter: Spider silk as archetypal protein elastomer. Soft Matter 2, 377 (2006).
X.Y. Liu, A. Sponner, D. Porter, and F. Vollrath: Proline and processing of spider silks. Biomacromolecules 9, 116 (2008).
K.N. Savage and J.M. Gosline: The role of proline in the elastic mechanism of hydrated spider silks. J. Exp. Biol. 211, 1948 (2008).
K.N. Savage and J.M. Gosline: The effect of proline on the network structure of major ampullate silks as inferred from their mechanical and optical properties. J. Exp. Biol. 211, 1937 (2008).
C.P. Brown, J. MacLeod, H. Amenitsch, F. Cacho-Nerin, H.S. Gill, A.J. Price, E. Traversa, S. Licoccia, and F. Rosei: The critical role of water in spider silk and its consequence for protein mechanics. Nanoscale 3, 3805 (2011).
J. Guan, D. Porter, and F. Vollrath: Silks cope with stress by tuning their mechanical properties under load. Polymer 53, 2717 (2012).
D. Porter, F. Vollrath, and Z. Shao: Predicting the mechanical properties of spider silk as a model nanostructured polymer. Eur. Phys. J. E: Soft Matter Biol. Phys. 16, 199 (2005).
B. Mortimer, S.D. Gordon, C. Holland, C.R. Siviour, F. Vollrath, and J.F.C. Windmill: The speed of sound in silk: Linking material performance to biological function. Adv. Mat. 26, 5179 (2014).
S. Keten and M.J. Buehler: Strength limit of entropic elasticity in beta-sheet protein domains. Phys. Rev. E 78, 061913 (2008).
S. Keten and M.J. Buehler: Atomistic model of the spider silk nanostructure. Appl. Phys. Lett. 96, 153701 (2010).
S. Keten, Z. Xu, B. Ihle, and M.J. Buehler: Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 9, 359 (2010).
Z. Qin and M.J. Buehler: Cooperative deformation of hydrogen bonds in beta-strands and beta-sheet nanocrystals. Phys. Rev. E 82, 061906 (2010).
S. Keten and M.J. Buehler: Nanostructure and molecular mechanics of dragline spider silk protein assemblies. J. Roy. Soc. Interface 7, 1709 (2010).
Sandeep P. Patil, B. Markert, and F. Gräter: Rate-dependent behavior of the amorphous phase of spider dragline silk. Biophys. J. 106, 2511 (2014).
T. Giesa, M. Arslan, N.M. Pugno, and M.J. Buehler: Nanoconfinement of spider silk fibrils begets superior strength, extensibility, and toughness. Nano Lett. 11, 5038 (2011).
S.W. Cranford: Increasing silk fibre strength through heterogeneity of bundled fibrils. J. R. Soc. Interface 10, 20130148 (2013).
G. Xu, L. Gong, Z. Yang, and X.Y. Liu: What makes spider silk fibers so strong? From molecular-crystallite network to hierarchical network structures. Soft Matter 10, 2116 (2014).
S.T. Krishnaji, G. Bratzel, M.E. Kinahan, J.A. Kluge, C. Staii, J.Y. Wong, M.J. Buehler, and D.L. Kaplan: Sequence–structure–property relationships of recombinant spider silk proteins: Integration of biopolymer design, processing, and modeling. Adv. Funct. Mater. 23, 241 (2013).
J.Y. Wong, J. McDonald, M. Taylor-Pinney, D.I. Spivak, D.L. Kaplan, and M.J. Buehler: Materials by design: Merging proteins and music. Nano Today 7, 488 (2012).
C. Holland, F. Vollrath, A.J. Ryan, and O.O. Mykhaylyk: Silk and synthetic polymers: Reconciling 100 degrees of separation. Adv. Mater. 24, 105 (2012).
F. Vollrath, D. Porter, and C. Holland: There are many more lessons still to be learned from spider silks. Soft Matter 7, 9595 (2011).
A Heidebrecht and T. Scheibel: Recombinant production of spider silk proteins. Adv. Appl. Microbiol. 82, 115 (2013).
N.A. Ayoub, J.E. Garb, A. Kuelbs, and C.Y. Hayashi: Ancient properties of spider silks revealed by the complete gene sequence of the prey-wrapping silk protein (AcSp1). Mol. Biol. Evol. 30, 589 (2013).
A. Chinali, W. Vater, B. Rudakoff, A. Sponner, E. Unger, F. Grosse, K.H. Guehrs, and K. Weisshart: Containment of extended length polymorphisms in silk proteins. J. Mol. Evol. 70, 325 (2010).
N.A. Ayoub, J.E. Garb, R.M. Tinghitella, M.A. Collin, and C.Y. Hayashi: Blueprint for a high-performance biomaterial: Full-length spider dragline silk genes. PLoS One 2, e514 (2007).
Y. Zhang, A.C. Zhao, Y.H. Sima, C. Lu, Z.H. Xiang, and M. Nakagaki: The molecular structures of major ampullate silk proteins of the wasp spider, Argiope bruennichi: A second blueprint for synthesizing de novo silk. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 164, 151 (2013).
K.W. Sanggaard, J.S. Bechsgaard, X. Fang, J. Duan, T.F. Dyrlund, V. Gupta, X. Jiang, L. Cheng, D. Fan, Y. Feng, L. Han, Z. Huang, Z. Wu, L. Liao, V. Settepani, I.B. Thogersen, B. Vanthournout, T. Wang, Y. Zhu, P. Funch, J.J. Enghild, L. Schauser, S.U. Andersen, P. Villesen, M.H. Schierup, T. Bilde, and J. Wang: Spider genomes provide insight into composition and evolution of venom and silk. Nat. Commun. 5, 3765 (2014).
P.L. Tai, G.Y. Hwang, and I.M. Tso: Inter-specific sequence conservation and intra-individual sequence variation in a spider silk gene. Int. J. Biol. Macromol. 34, 295 (2004).
R. Beckwitt and S. Arcidiacono: Sequence conservation in the C-terminal region of spider silk proteins (Spidroin) from Nephila clavipes (tetragnathidae) and Araneus bicentenarius (Araneidae). J. Biol. Chem. 269, 6661 (1994).
G. Askarieh, M. Hedhammar, K. Nordling, A. Saenz, C. Casals, A. Rising, J. Johansson, and S.D. Knight: Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 465, 236 (2010).
G. Gronau, Z. Qin, and M.J. Buehler: Effect of sodium chloride on the structure and stability of spider silk’s N-terminal protein domain. Biomater. Sci. 1, 276 (2013).
F. Hagn, L. Eisoldt, J.G. Hardy, C. Vendrely, M. Coles, T. Scheibel, and H. Kessler: A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 465, 239 (2010).
F. Prosdocimi, D. Bittencourt, F.R. da Silva, M. Kirst, P.C. Motta, and E.L. Rech: Spinning gland transcriptomics from two main clades of spiders (order: Araneae)—Insights on their molecular, anatomical and behavioral evolution. PLoS One 6, e21634 (2011).
T.H. Clarke, J.E. Garb, C.Y. Hayashi, R.A. Haney, A.K. Lancaster, S. Corbett, and N.A. Ayoub: Multi-tissue transcriptomics of the black widow spider reveals expansions, co-options, and functional processes of the silk gland gene toolkit. BMC Genomics 15, 365 (2014).
R.F. Foelix: The Biology of Spiders (Oxford University Press, New York, 1996). p. 336.
T. Lefèvre, S. Boudreault, C. Cloutier, and M. Pézolet: Diversity of molecular transformations involved in the formation of spider silks. J. Mol. Biol. 405, 238 (2011).
D. Knight and F. Vollrath: Hexagonal columnar liquid crystal in the cells secreting spider silk. Tissue Cell 31, 617 (1999).
D.H. Hijirida, K.G. Do, C. Michal, S. Wong, D. Zax, and L.W. Jelinski: 13C NMR of Nephila clavipes major ampullate silk gland. Biophys. J. 71, 3442 (1996).
H.J. Jin and D.L. Kaplan: Mechanism of silk processing in insects and spiders. Nature 424, 1057 (2003).
S. Rammensee, U. Slotta, T. Scheibel, and A.R. Bausch: Assembly mechanism of recombinant spider silk proteins. Proc. Natl. Acad. Sci. U. S. A. 105, 6590 (2008).
F. Vollrath, N. Hawkins, D. Porter, C. Holland, and M. Boulet-Audet: Differential scanning fluorimetry provides high throughput data on silk protein transitions. Sci. Rep. 4, 5625 (2014).
J.G. Hardy, L.M. Römer, and T.R. Scheibel: Polymeric materials based on silk proteins. Polymer 49, 4309 (2008).
F. Vollrath and D.P. Knight: Structure and function of the silk production pathway in the spider Nephila edulis. Int. J Biol. Macromol. 24, 243 (1999).
J. Leclerc, T. Lefèvre, M. Gauthier, S.M. Gagné, and M. Auger: Hydrodynamical properties of recombinant spider silk proteins: Effects of pH, salts and shear, and implications for the spinning process. Biopolymers 99, 582 (2013).
J. Kovoor and A. Munoz-Cuevas: Structure and function of the silk-gland system in Oxyopidae (Araneae). In Proceedings of the 17th European Colloquium of Arachnology, Edinburgh 1997, 1998; p. 133.
M.A. Garrido, M. Elices, C. Viney, and J. Pérez-Rigueiro: Active control of spider silk strength: Comparison of drag line spun on vertical and horizontal surfaces. Polymer 43, 1537 (2002).
A.A. Griffith: The phenomena of rupture and flow in solids. Phil. Trans. R. Soc. A 221, 163 (1921).
F. Teulé, A.R. Cooper, W.A. Furin, D. Bittencourt, E.L. Rech, A. Brooks, and R.V. Lewis: A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nat. Protoc. 4, 341 (2009).
R. Menassa, H. Zhu, C.N. Karatzas, A. Lazaris, A. Richman, and J. Brandle: Spider dragline silk proteins in transgenic tobacco leaves: Accumulation and field production. Plant Biotechnol. J. 2, 431 (2004).
S.R. Fahnestock and L.A. Bedzyk: Production of synthetic spider dragline silk protein in Pichia pastoris. Appl. Microbiol. Biotechnol. 47, 33 (1997).
F. Teulé, Y-G. Miao, B-H. Sohn, Y-S. Kim, J.J. Hull, M.J. Fraser, R.V. Lewis, and D.L. Jarvis: Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. U.S.A. 109, 923 (2012).
H.B. Steinkraus, H. Rothfuss, J.A. Jones, E. Dissen, E. Shefferly, and R.V. Lewis: The absence of detectable fetal microchimerism in nontransgenic goats (Capra aegagrus hircus) bearing transgenic offspring. J. Anim. Sci. 90, 481 (2012).
A. Lazaris, S. Arcidiacono, Y. Huang, J-F. Zhou, F. Duguay, N. Chretien, E.A. Welsh, J.W. Soares, and C.N. Karatzas: Spider silk fibers spun from soluble recombinant silk produced in mammalian cells. Science 295, 472 (2002).
J. Leclerc, T. Lefèvre, F. Pottier, L.P. Morency, C. Lapointe-Verreault, S.M. Gagné, and M. Auger: Structure and pH-induced alterations of recombinant and natural spider silk proteins in solution. Biopolymers 97, 337 (2012).
X-X. Xia, Z-G. Qian, C.S. Ki, Y.H. Park, D.L. Kaplan, and S.Y. Lee: Native-sized recombinant spider silk protein produced in metabolically engineered Escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. U. S. A. 107, 14059 (2010).
S.R. Fahnestock, Z. Yao, and L.A. Bedzyk: Microbial production of spider silk proteins. Rev. Mol. Biotechnol. 74, 105 (2000).
D.M. Widmaier, D. Tullman-Ercek, E.A. Mirsky, R. Hill, S. Govindarajan, J. Minshull, and C.A. Voigt: Engineering the Salmonella type III secretion system to export spider silk monomers. Mol. Syst. Biol. 5, 308 (2009).
D.M. Widmaier and C.A. Voigt: Quantification of the physiochemical constraints on the export of spider silk proteins by Salmonella type III secretion. Microb. Cell Fact. 9, 78 (2010).
A.M. Goncalves, A.Q. Pedro, C. Maia, F. Sousa, J.A. Queiroz, and L.A. Passarinha: Pichia pastoris: A recombinant microfactory for antibodies and human membrane proteins. J. Microbiol. Biotechnol. 23, 587 (2013).
V. Hauptmann, N. Weichert, M. Rakhimova, and U. Conrad: Spider silks from plants—A challenge to create native-sized spidroins. Biotechnol. J. 8, 1183 (2013).
S. Grip, A. Rising, H. Nimmervoll, E. Storckenfeldt, S.J. Mcqueen-Mason, N. Pouchkina-Stantcheva, F. Vollrath, W. Engström, and A. Fernandez-Arias: Transient expression of a major ampullate spidroin 1 gene fragment from Euprosthenops sp. in mammalian cells. Cancer Genom. Proteom. 3, 83 (2006).
N. Weichert, V. Hauptmann, M. Menzel, K. Schallau, P. Gunkel, T.C. Hertel, M. Pietzsch, U. Spohn, and U. Conrad: Transglutamination allows production and characterization of native-sized ELPylated spider silk proteins from transgenic plants. Plant Biotechnol. J. 12, 265 (2014).
H-T. Xu, B-L. Fan, S-Y. Yu, Y-H. Huang, Z-H. Zhao, Z-X. Lian, Y-P. Dai, L-L. Wang, Z-L. Liu, J. Fei, and N. Li: Construct synthetic gene encoding artificial spider dragline silk protein and its expression in milk of transgenic mice. Ani. Biotechnol. 18, 1 (2007).
M. Elices, G.V. Guinea, G.R. Plaza, C. Karatzas, C. Riekel, F. Agulló-Rueda, R. Daza, and J. Pérez-Rigueiro: Bioinspired fibers follow the track of natural spider silk. Macromolecules 44, 1166 (2011).
Y. Zhang, J. Hu, Y. Miao, A. Zhao, T. Zhao, D. Wu, L. Liang, A. Miikura, K. Shiomi, Z. Kajiura, and M. Nakagaki: Expression of EGFP-spider dragline silk fusion protein in BmN cells and larvae of silkworm showed the solubility is primary limit for dragline proteins yield. Mol. Biol. Rep. 35, 329 (2008).
K. Schacht and T. Scheibel: Processing of recombinant spider silk proteins into tailor-made materials for biomaterials applications. Curr. Opin. Biotechnol. 29, 62 (2014).
P. Domachuk, K. Tsioris, F.G. Omenetto, and D.L. Kaplan: Bio-microfluidics: Biomaterials and biomimetic designs. Adv. Mater. 22, 249 (2010).
H. Daniel and S. Thomas: Method and device for producing a thread from silk proteins. U.S. Patent No. 7,868,146. 11 January 2011.
D.P. Knight and L. Pinnock: Method and apparatus for forming objects. WO Patent App. PCT/EP2003/014,787. 8 July 2004.
M.E. Kinahan, E. Filippidi, S. Köster, X. Hu, H.M. Evans, T. Pfohl, D.L. Kaplan, and J. Wong: Tunable silk: Using microfluidics to fabricate silk fibers with controllable properties. Biomacromolecules 12, 1504 (2011).
J. Luo, L. Zhang, Q. Peng, M. Sun, Y. Zhang, H. Shao, and X. Hu: Tough silk fibers prepared in air using a biomimetic microfluidic chip. Int. J Biol. Macromol. 66, 319 (2014).
G.J.G. Davies, D.P. Knight, and F. Vollrath: Structure and function of the major ampullate spinning duct of the golden orb weaver, Nephila edulis. Tissue Cell 45, 306 (2013).
B. Renberg, H. Andersson-Svahn, and M. Hedhammar: Mimicking silk spinning in a microchip. Sens. Actuators B 195, 404 (2014).
C. Holland, A.E. Terry, D. Porter, and F. Vollrath: Natural and unnatural silks. Polymer 48, 3388 (2007).
X. Chen, D.P. Knight, and F. Vollrath: Rheological characterization of Nephila spidroin solution. Biomacromolecules 3, 644 (2002).
F. Vollrath, D.P. Knight, and X.W. Hu: Silk production in a spider involves acid bath treatment. Phil. Trans. R. Soc. B 265, 817 (1998).
Z. Shao, F. Vollrath, Y. Yang, and H.C. Thøgersen: Structure and behavior of regenerated spider silk. Macromolecules 36, 1157 (2003).
A. Seidel, O. Liivak, S. Calve, J. Adaska, G. Ji, Z. Yang, D. Grubb, D.B. Zax, and L.W. Jelinski: Regenerated spider silk: Processing, properties, and structure. Macromolecules 33, 775 (2000).
S. Inoue, K. Tanaka, F. Arisaka, S. Kimura, K. Ohtomo, and S. Mizuno: Silk fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 275, 40517 (2000).
D.N. Breslauer, L.P. Lee, and S.J. Muller: Simulation of flow in the silk gland. Biomacromolecules 10, 49 (2008).
D. Porter, J. Guan, and F. Vollrath: Spider silk: Super material or thin fibre?Adv. Mater. 25, 1275 (2013).
Z. Huang, Y. Lu, R. Majithia, J. Shah, K. Meissner, K.S. Matthews, S.E. Bondos, and J. Lou: Size dictates mechanical properties for protein fibers self-assembled by the Drosophila hox transcription factor ultrabithorax. Biomacromolecules 11, 3644 (2010).
J. Smook, W. Hamersma, and A.J. Pennings: The fracture process of ultra-high strength polyethylene fibres. J. Mat. Sci. 19, 1359 (1984).
T. Amornsakchai, D. Cansfield, S. Jawad, G. Pollard, and I. Ward: The relation between filament diameter and fracture strength for ultra-high-modulus polyethylene fibres. J. Mat. Sci. 28, 1689 (1993).
H.D. Wagner: Dependence of fracture stress upon diameter in strong polymeric fibers. J. Macromol. Sci. Phys. 28, 339 (1989).
H. Wagner: Stochastic concepts in the study of size effects in the mechanical strength of highly oriented polymeric materials. J. Polym. Sci. Part B Polym. Phys. 27, 115 (1989).
H.G. Chae, Y.H. Choi, M.L. Minus, and S. Kumar: Carbon nanotube reinforced small diameter polyacrylonitrile based carbon fiber. Composites Sci. Technol. 69, 406 (2009).
Y. Ji, B. Li, S. Ge, J.C. Sokolov, and M.H. Rafailovich: Structure and nanomechanical characterization of electrospun PS/clay nanocomposite fibers. Langmuir 22, 1321 (2006).
K. Young, F.M. Blighe, J.J. Vilatela, A.H. Windle, I.A. Kinloch, L. Deng, R.J. Young, and J.N. Coleman: Strong dependence of mechanical properties on fiber diameter for polymer–nanotube composite fibers: Differentiating defect from orientation effects. ACS Nano 4, 6989 (2010).
D. Mackenzie: The history of sutures. Med. Hist. 17, 158 (1973).
K. Gellynck, P. Verdonk, R. Forsyth, K.F. Almqvist, E. Van Nimmen, T. Gheysens, J. Mertens, L. Van Langenhove, P. Kiekens, and G. Verbruggen: Biocompatibility and biodegradability of spider egg sac silk. J. Mater. Sci. Mater. Med. 19, 2963 (2008).
F. Vollrath: Strength and structure of spiders’ silks. J. Biotechnol. 74, 67 (2000).
G.H. Altman, F. Diaz, C. Jakuba, T. Calabro, R.L. Horan, J. Chen, H. Lu, J. Richmond, and D.L. Kaplan: Silk-based biomaterials. Biomaterials 24, 401 (2003).
L. Meinel, S. Hofmann, V. Karageorgiou, C. Kirker-Head, J. McCool, G. Gronowicz, L. Zichner, R. Langer, G. Vunjak-Novakovic, and D.L. Kaplan: The inflammatory responses to silk films in vitro and in vivo. Biomaterials 26, 147 (2005).
J.G. Hardy and T.R. Scheibel: Composite materials based on silk proteins. Prog. Polym. Sci. 35, 1093 (2010).
D.N. Rockwood, R.C. Preda, T. Yücel, X. Wang, M.L. Lovett, and D.L. Kaplan: Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6, 1612 (2011).
H-J. Jin, J. Chen, V. Karageorgiou, G.H. Altman, and D.L. Kaplan: Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 25, 1039 (2004).
R. Nazarov, H-J. Jin, and D.L. Kaplan: Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 5, 718 (2004).
S. Hofmann, C. Wong Po Foo, F. Rossetti, M. Textor, G. Vunjak-Novakovic, D. Kaplan, H. Merkle, and L. Meinel: Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 111, 219 (2006).
K.D. Hermanson, D. Huemmerich, T. Scheibel, and A.R. Bausch: Engineered microcapsules fabricated from reconstituted spider silk. Adv. Mater. 19, 1810 (2007).
S.C. Gomes, I.B. Leonor, J.F. Mano, R.L. Reis, and D.L. Kaplan: Antimicrobial functionalized genetically engineered spider silk. Biomaterials 32, 4255 (2011).
D-H. Kim, J. Viventi, J.J. Amsden, J. Xiao, L. Vigeland, Y-S. Kim, J.A. Blanco, B. Panilaitis, E.S. Frechette, and D. Contreras: Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511 (2010).
S. Kim, A.N. Mitropoulos, J.D. Spitzberg, H. Tao, D.L. Kaplan, and F.G. Omenetto: Silk inverse opals. Nat. Photonics 6, 818 (2012).
J. MacLeod and F. Rosei: Photonic crystals: Sustainable sensors from silk. Nat. Mater. 12, 98 (2013).
Y.Y. Diao, X.Y. Liu, G.W. Toh, L. Shi, and J. Zi: Multiple structural coloring of silk-fibroin photonic crystals and humidity-responsive color sensing. Adv. Funct. Mat. 23, 5373 (2013).
B.D. Lawrence, M. Cronin-Golomb, I. Georgakoudi, D.L. Kaplan, and F.G. Omenetto: Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules 9, 1214 (2008).
J.J. Amsden, H. Perry, S.V. Boriskina, A. Gopinath, D.L. Kaplan, L. Dal Negro, and F.G. Omenetto: Spectral analysis of induced color change on periodically nanopatterned silk films. Opt. Express 17, 21271 (2009).
J.J. Amsden, P. Domachuk, A. Gopinath, R.D. White, L.D. Negro, D.L. Kaplan, and F.G. Omenetto: Rapid nanoimprinting of silk fibroin films for biophotonic applications. Adv. Mater. 22, 1746 (2010).
X. Huang, G. Liu, and X. Wang: New secrets of spider silk: Exceptionally high thermal conductivity and its abnormal change under stretching. Adv. Mater. 24, 1482 (2012).
R. Fuente, A. Mendioroz, and A. Salazar: Revising the exceptionally high thermal diffusivity of spider silk. Mater. Lett. 114, 1 (2014).
L. Zhang, T. Chen, H. Ban, and L. Liu: Hydrogen bonding-assisted thermal conduction in β-sheet crystals of spider silk protein. Nanoscale 6, 7786 (2014).
B. Tulachan, S.K. Meena, R.K. Rai, C. Mallick, T.S. Kusurkar, A.K. Teotia, N.K. Sethy, K. Bhargava, S. Bhattacharya, A. Kumar, R.K. Sharma, N. Sinha, S.K. Singh, and M. Das: Electricity from the silk cocoon membrane. Sci. Rep. 4, 5434 (2014).
C.P. Brown, F. Rosei, E. Traversa, and S. Licoccia: Spider silk as a load-bearing biomaterial: Tailoring mechanical properties via structural modifications. Nanoscale 3, 870 (2011).
R.W. Work: Viscoelastic behaviour and wet supercontraction of major ampullate silk fibres of certain orb-web-building spiders (Araneae). J. Exp. Biol. 118, 379 (1985).
Y. Liu, Z. Shao, and F. Vollrath: Relationships between supercontraction and mechanical properties of spider silk. Nat. Mater. 4, 901 (2005).
ACKNOWLEDGMENTS
This work was supported by Arthritis Research UK (C.P.B), the University of the Sunshine Coast, Australia (C.P.B. and J.M.), and the Natural Sciences and Engineering Research Council of Canada (F.R.). C.P.B. is grateful to Arthritis Research UK for salary support. F.R. is grateful to the Canada Research Chairs program for partial salary support, thanks the Alexander von Humboldt Foundation for a F.W. Bessel Award, and acknowledges NSERC for an EWR Steacie Memorial Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Brown, C.P., Whaite, A.D., MacLeod, J.M. et al. With great structure comes great functionality: Understanding and emulating spider silk. Journal of Materials Research 30, 108–120 (2015). https://doi.org/10.1557/jmr.2014.365
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/jmr.2014.365