Abstract
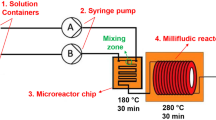
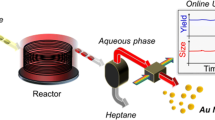
Cobalt nanoparticles were synthesized using continuous-flow (CF) chemistry in a stainless steel microreactor for the first time at high output based on the ethanol hydrazine alkaline system (EHAS) producing a yield as high as 1 g per hour [1, 2]. Continuous-flow (CF) synthetic chemistry provides uninterrupted product formation allowing for advantages including decreased preparation time, improved product quality, and greater efficiency. This successful synthetic framework in continuous-flow of magnetic Co nanoparticles indicates feasibility for scaled-up production. The average particle size by transmission electron microscopy (TEM) of the as-synthesized cobalt was 30±10 nm, average crystallite size by Scherrer analysis (fcc phase) was 15±2 nm, and the estimated magnetic core size was 6±1 nm. Elemental surface analysis (X-ray photoelectron spectroscopy [XPS]) indicates a thin CoO surface layer. Assynthesized cobalt nanoparticles possessed a saturation magnetization (Ms) of 125±1 emu/g and coercivity (Hc) of 120±5 Oe. The actual Ms is expected to be greater since the as-synthesized cobalt mass was not weight-corrected (nonmagnetic mass: reaction by-products, solvent, etc.). Our novel high-output, continuous-flow production (>1 g/hr) of highly magnetic cobalt nanoparticles opens an avenue toward industrial-scale production of several other single element magnetic nanomaterials.
Similar content being viewed by others
References
Guo, F.; Zheng, H.; Yang, Z.; Qian, Y. Mater. Lett. 2002, 56, 906.
Gibson, C. P.; Putzer, K. J. Science 1995, 267, 1338.
El-Gendy, A. A.; Ibrahim, E. M. M.; Khavrus, V. O.; Krupskaya, Y.; Hampel, S.; Leonhardt, A.; Büchner, B.; Klingeler, R. Carbon 2009, 47, 2821.
Majewski, A. P.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Müller, A. H. E.; Schmalz, H. Biomacromolecules 2013, 14, 3081.
Li, Y.; Beija, M.; Laurent, S.; Elst, L. V.; Muller, R. N.; Duong, H. T. T.; Lowe, A. B.; Davis, T. P.; Boyer, C. Macromolecules 2012, 45, 4196.
Osorio-Cantillo, C.; Santiago-Miranda, A. N.; Perales-Perez, O.; Yin, X. J. Appl. Phys. 2012, 111,07B324.
Giesen, B.; Orthner, H. R.; Kowalik, A.; Roth, P. Chem. Eng. Sci. 2004, 59, 2201.
LaLena, J. N.; Cleary, D. A.; Carpenter, E. E.; Dean, N. F. Inorganic Materials Synthesis and Fabrication; Wiley Interscience: Hoboken, NJ, 2008.
McMullen, J. P.; Jensen K. F. Annu. Rev. Anal. Chem. 2010, 3, 19.
Razzaq, T.; Glasnov, T.N.; Kappe, C. O. Eur. J. Org. Chem. 2009, 9, 1321.
Hassan, A. A.; Sandre, O.; Cabuil, V. Angew. Chem., Int. Ed. 2010, 49, 6268.
Marre, S.; Jensen, K. F. Chem. Soc. Rev. 2010, 39, 1183.
Shalom, D.; Wootton, R. C. R.; Winkle, R. F.; Cottam, B. F.; Vilar, R.; deMello, A. J.; Wilde, C. P. Mater. Lett. 2007, 61, 1146.
Weng, C. H.; Huang, C. C.; Yeh, C. S.; Lee, H. Y.; Lee, G. B. J. Micromech. Microeng. 2008, 18, 035019.
Shestopalov, I; Tice, J. D.; Ismagilov, R. F. Lab Chip 2004, 4, 316.
Chen, C. H.; Shah, R. K.; Abate, A. R. Weitz, D. A. Langmuir 2009, 25, 4320.
Gross, E; Shu, X.-Z.; Alayoglu, S.; Bechtel, H. A. Martin, M. C. Toste, F. D.; Somorjai, G. A. J. Am. Chem. Soc. 2014, 136, 3624.
Hassan, A. A.; Sandre, O.; Cabuil, V.; Tabeling P. Chem. Commun. 2008, 1783.
Hassan, A. A.; Sandre, O.; Neveu, S.; Cabuil, V. Angew. Chem., Int. Ed. 2009, 48, 2342.
Bubendor, J. L.; Enyb, C. M.; Beaurepaire, E.; Panissod, P.; Bucher, J. P. Eur. Phys. J. B. 2000, 17, 635.
De la Peña-O’Shea, V. A.; Moreira, I. P. R.; Roldán; A.; Illas, F. J. Chem. Phys. 2010, 133, 024701.
Barr, T. L. J. Phys. Chem. 1978, 82, 1801.
Crist, V. Handbook of Monochromatic XPS Spectra — The Elements and Native Oxides; XPS International Inc.: Mountain View, CA, 1999; vol. 1.
Biesinger, M. C.; Payne, B. P.; Grosvenor, A. P.; Lau, L. W. M; Gerson, A. R. St. C. J. Appl. Surf. Sci. 2011, 257, 2717.
Yaacob, I. I.; Nunes, A. C.; Bose, A.; Shah, D. O. J. Colloid Interface Sci. 1994, 168, 289.
Crangle, J. TheMagneticProperties of Solids; Edward Arnold: London, 1977.
Carpenter, E. E. J. Magn. Magn. Mater. 2001, 225, 17.
El-Gendy, A. A.; Khavrus, V. O.; Hampel, S.; Leonhardt A.; Büchner, B.; Klingeler, R. J. Phys. Chem. C 2010, 114, 10745.
Glaspell, G.; Abdelsayed, V.; Saoud, K. M.; El-Shall, S. Pure Appl. Chem. 2006, 78, 1667.
Deepak, S.; Chauhan, R.; Kumar, S. J. Mater. Sci. 2014, 25, 124.
Liu, T.; Zhou, P. H.; Xie, J. L.; Deng, L. J. J. Appl. Phys. 2011, 110, 033918.
Kammel, M.; Wiedenmann, A.; Heinemann, A.; Bönnemann, H.; Matoussevitch, N. Phys. B: Phys. Condens. Matter 2006, 385, 457.
Desvaux, C.; Amiensl, C.; Fejes, P.; Renaud, P.; Respaud, M.; Lecante, P.; Snoeck, E.; Chaudret, B. Nat. Mater. 2005, 4, 750.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Clifford, D.M., El-Gendy, A.A., Lu, A.J. et al. Room Temperature Synthesis of Highly Magnetic Cobalt Nanoparticles by Continuous Flow in a Microfluidic Reactor. J Flow Chem 4, 148–152 (2014). https://doi.org/10.1556/JFC-D-14-00013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1556/JFC-D-14-00013