Abstract
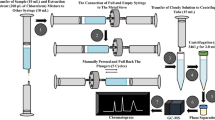
Pharmacy staff and health care workers in hospitals may be exposed to antineoplastic drugs during cancer chemotherapy. Sensitive methods should be used to monitor the occupational exposure in biofluids such as urine. In this study, a sensitive method for cyclophosphamide determination in urine with high recovery was developed and validated for monitoring occupational exposure. Triple liquid-liquid extraction with ethyl acetate/dichloromethane (φr= 3:1; 6.0 mL) was applied. Good separation of cyclophosphamide and ifosfamide was achieved in a 9.0 min analysis, using liquid chromatography combined with tandem mass spectrometry (LC-MS/MS). Limit of detection and limit of quantification were 0.07 ng mL−1 and 0.11 ng mL−1, respectively. Repeatability (RSD, %) was ≤ 12.74%. Inter-day and inter-analyst precision values (RSD, %) were ≤ 13.94% and 12.59%. Mean recovery for three different concentrations was (101.62 ± 6.05) %. The validation results were fitted for the purpose of routine monitoring of occupational cyclophosphamide exposure in hospital staff.
Similar content being viewed by others
References
AOAC International (2012). Appendix F: guidelines for standard method performance requirements. Retrieved June 2, 2015, from http://www.eoma.aoac.org/app_f.pdf
B’Hymer, C., & Cheever, K. L. (2010). Evaluation of a procedure for the simultaneous quantification of 4-ketocyclophosphamide, cyclophosphamide, and ifosfamide in human urine. Journal of Chromatographic Science, 48 328–333. DOI: 10.1093/chromsci/48.5.328.
Cserháti, T., & Szögyi, M. (2013). Chromatography of anti-cancer drugs. European Chemical Bulletin, 2 715–721.
Dhakane, V. D., & Ubale, M. B. (2013). Development and validation of a reverse phase high performance liquid chromatographic method for the estimation of cyclophosphamide in bulk drug. International Journal of Pharmacy & Pharmaceutical Sciences, 5 184–187.
Eisenberg, S. (2009). Safe handling and administration of antineoplastic chemoterapy. Journal of Infusion Nursing, 32 23–32. DOI: 10.1097/nan.0b013e31819246e0.
Eurachem (1998). The fitness for purpose of analytical methods: a laboratory guide to method validation and related topics. Retrieved June 2, 2015, from http://eurachem.org/images/stories/Guides/pdf/valid.pdf
Fransman, W., Kager, H., Meijster, T., Heederik, D., Kromhout, H., Portengen, L., & Blaauboer, B. J. (2014). Leukemia from dermal exposure to cyclophosphamide among nurses in the Netherlands: Quantitative assessment of the risk. The Annals of Occupational Hygiene, 58 271–282. DOI: 10.1093/annhyg/met077.
Hedmer, M., Georgiadi, A., Bremberg, E. R., Jönsson, B. A. G., & Eksborg, S. (2005). Surface contamination of cyclophosphamide packaging and surface contamination with antineoplastic drugs in a hospital pharmacy in Sweden. Annals of Occupational Hygiene, 49 629–637. DOI: 10.1093/annhyg/mei042.
Hirst, M., Mills, D., Tse, S., Levin, L., & White, D. F. (1984). Occupational exposure to cyclophosphamide. The Lancet, 323 186–188. DOI: 10.1016/s0140-6736(84)92111-1.
Ghante, M. R., Pannu, H. K., Loni, A., & Shivsharan, T. (2012). Development and validation of a RP-HPLC method for simultaneous estimation of metronidazole and norfloxacin in bulk and tablet dosage form. International Journal of Pharmacy & Pharmaceutical Sciences, 4 241–245.
Karahalil, B., & Akkoyunlu, K. I. (2003). Determination of urinary cyclophosphamide in oncology nurses handling antineoplastic drugs by gas chromatography-mass spectrometry. FABAD Journal of Pharmaceutical Sciences, 28 125–130.
Kasel, D., Jetter, A., Harlfinger, S., Gebhardt, W., & Fuhr, U. (2004). Quantification of cyclophosphamide and its metabolites in urine using liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 18 1472–1478. DOI: 10.1002/rcm.1508.
Minioa, C., Turci, R., Sottani, C., Schiavi, A., Perbellini, L., Angeleri, S., Draicchio, F., & Apostoli, P. (1998). Application of high performance liquid chromatography/tandem mass spectrometry in the environmental and biological monitoring of health care personel occupationally exposed to cyclophosphamide and ifosfamide. Rapid Communications in Mass Spectrometry, 12 1485–1493. DOI: 10.1002/(SICI)1097-0231(19981030)12:20<1485∷AID-RCM333>3.0.CO;2-N.
Mocak, J., Bond, A. M., Mitchell, S., & Scollary, G. (1997). A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: application to voltammetric and stripping techniques. Pure and Applied Chemistry, 69 297–328. DOI: 10.1351/pac199769020297.
Pethran, A., Schierl, R., Hauff, K., Grimm, C. H., Boos, K. S., & Nowak, D. (2003). Uptake of antineoplastic agents in pharmacy and hospital personel. Part 1: Monitoring of urinary concentration. International Archives of Occupational and Environmental Health, 76 5–10. DOI: 10.1007/s00420-002-0383-8.
Rekhadevi, P. V., Sailaja, N., Chandrasekhar, M., Mahboob, M., Rahman, M. F., & Grover, P. (2007). Genotoxicity assessment in oncology nurses handling anti-neoplastic drugs. Mutagenesis, 22 395–401. DOI: 10.1093/mutage/gem032.
Rivera, C., & Rodríguez, R. (2014). Horwitz equation as quality benchmark in ISO/IEC 17025 testing laboratory. Retrieved June 2, 2015, from http://www.bii.mx/documentos/horwitzCf11.pdf
Ruta, J., Zurlino, D., Grivel, C., Heinisch, S., Veuthey, J. L., & Guillarme, D. (2012). Evaluation of columns packed with shell particles with compounds of pharmaceutical interest. Journal of Chromatography A, 1228 221–231. DOI: 10.1016/j.chroma.2011.09.013.
Sato, J., Mori, M., Sasaki, T., Nihei, S., Kumagai, M., Nakayama, S., & Kudo, K. (2014). Field survey of the anti-cancer drug contamination in the preparation environment-usefulness of the 5-FU monitoring by the coupon method. Journal of the Pharmaceutical Society of Japan, 134 751–756. DOI: 10.1248/yakushi.13-00154.
Schreiber, C., Radon, K., Pethran, A., Schierl, R., Hauff, K., Grimm, C. H., Boos, K. S., & Nowak, D. (2003). Uptake of antineoplastic agents in pharmacy personnel. Part II: study of work-related risk factors. International Archives of Occupational and Environmental Health, 76 11–16. DOI: 10.1007/s00420-002-0385-6.
Sertler, S. (2013). Assessment of the safe working condition of the healthcare personnel with antineoplastic drugs and biological and environmental contamination, Ph.D. Thesis, University of Istanbul, Istanbul, Turkey.
Sessink, P. J. M., Cerná, M., Rössner, P., Pastorková, A., Bavarová, H., Franková, K., Anzion, R. B. M., & Bos, R. P. (1994). Urinary cyclophosphamide excretion and chromosomal aberrations in peripheral blood lymphocytes after occupational exposure to antineoplastic agents. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 309 193–199. DOI: 10.1016/0027-5107(94)90092-2.
Sottani, C., Turci, R., Perbellini, L., & Minioa, C. (1998). Liquid-liquid extraction procedure for trace determination of cyclophosphamide in human urine by high-performance liquid chromatography tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 12 1063–1068. DOI: 10.1002/(SICI)1097-0231(19980831)12:16<1063∷AID-RCM287>3.0.CO;2-K.
Sottani, C., Tranfo, G., Faranda, P., & Minoia, C. (2005). Highly sensitive high-performance liquid chromatography/selective reaction monitoring mass spectrometry method for the determination of cyclophosphamide and ifosfamide in urine of health care workers exposed to antineoplastic agents. Rapid Communications in Mass Spectrometry, 19 2794–2800. DOI: 10.1002/rcm.2116.
Sottani, C., Rinaldi, P., Leoni, E., Poggi, G., Teragni, C., Delmonte, A., & Minoia, C. (2008). Simultaneous determination of cyclophosphamide, ifosfamide, doxorubicin, epirubicin and daunorubicin in human urine using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry: Bioanalytical method validation. Rapid Communications in Mass Spectrometry, 22 2645–2659. DOI: 10.1002/rcm.3657.
Tanimura, M., Yamada, K., Sugiura, S. I., Mori, K., Nagata, H., Tadokoro, K., Miyake, T., Hamaguchi, Y., Sessink, P., & Nabeshima, T. (2009). An environmental and biological study of occupational exposure to cyclophosphamide in the pharmacy of a Japanese community hospital designated for the treatment of cancer. Journal of Health Science, 55 750–756. DOI: 10.1248/jhs.55.750.
Ziegler, E., Mason, H. J., & Baxter, P. J. (2002). Occupational exposure to cytotoxic drugs in two UK oncology wards. Occupational and Environmental Medicine, 59 608–612. DOI: 10.1136/oem.59.9.608.
Zhang, J., Tian, Q., & Zhou, S. F. (2006). Clinical pharmacology of cyclophosphamide and ifosfamide. Current Drug Therapy, 1 55–84. DOI: 10.2174/157488506775268515.
Zhou, J. Y., Gao, S. H., Zhang, F., Jiang, B., Zhan, Q., Cai, F., Li, J. X., & Chen, W. S. (2012). Liquid chromatography-tandem mass spectrometry method for simultaneous determination of seven commonly used anticancer drugs in human plasma. Journal of Chromatography B, 906 1–8. DOI: 10.1016/j.jchromb.2012.07.033.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anılanmert, B., Sertler, S., Cavus, F. et al. Validated method for occupational cyclophosphamide monitoring using LC-MS/MS and a Poroshell 120 column. Chem. Pap. 69, 1395–1401 (2015). https://doi.org/10.1515/chempap-2015-0157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/chempap-2015-0157