Abstract
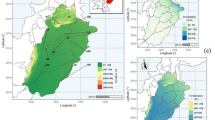
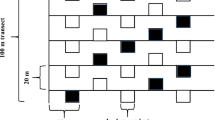
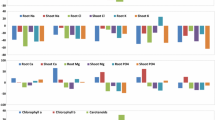
Three ecotypes [foot hill (700 m), mid hill (1571 m) and top hill (2804 m)] of a Bermuda grass Cynodon dactylon (L.) Pers. from Pir Chinasi Hill in Western Himalaya were evaluated for their degree of tolerance to altitudinal stress. Differential response of all ecotypes in terms of adequate structural modifications to different elevation leveis was an evident to confirm the hypothesis that plants inhabiting different altitudes show variation in structure (internal modifications) and strategic (response) due to heterogeneity in environmental gradients. Soil at top hill site was more acidic and displayed significant increase in ionic content and total nitrogen. High elevation had severe impact on morpho-anatomical and physiological attributes. A significant decline in shoot fresh weight and total leaf area was observed in top hill ecotype. With exception of Ca2+ and carotenoid, other ionic and chlorophyll content were significantly declined at high elevations. Anatomical alterations such as, increased leaf thickness, intensive sclerification around the vascular bundle and pith area, reduced metaxylem vessel area, high number of silica bodies, high pubescence (increased microhair and trichome density) were some of the promising anatomical adaptations in top hill ecotype which played an important role in high degree of tolerance of this grass to cope with altitudinal stresses. Increased leaf thickness might be a response to lower temperature that protects mesophyll cells and high density of trichomes may be involved in blocking transpiration water and internal heat. The pattern of constant variation suggests that differential response of these ecotypes is highly related to air temperature, pattern of rainfall, availability of nutrients.
Similar content being viewed by others
References
Alvarez J.M., Rocha J.F. & Machado S.R. 2008. Bulliform cells in Loudefaopsis chrysothrix (Nees) Conert and Tristachya leiostachya Nees (Poaceae): structure in relation to function Braz. Arch. Bio. Technol. 51: 113–119.
Arnon D.I. 1949. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 24: 1–15.
Atkin O.K. & Day D.D. 1990. A comparison of the respiratory processes and growth rates of selected Australian alpine and related lowland species. Aus. J. Plant Physiol. 17: 517–526
Baath E. & Anderson T.H. 2003 Comparison of soil fun-gal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 35: 955–963.
Beniston M. 2003. Climatic change in mountain regions: a review of possible impacts. Climatic Change 59: 5–31.
Cole V.C., Paustian K., Elliott E.T., Metherell A.K., Ojima D.S. & Parton W.J. 1993. Analysis of agroecosystem carbon pools. Water Air Soil Poli. 70: 357–371.
Fatemeh Z., Tajik S. & Soleimanpour S. 2011. Effects of alti-tude on anatomy and concentration of Crocin, Picrocrocin and Safranal in Crocus sativus L. Aus. J. Crop Sci. 5: 831–838.
Flann C., Ladiges P.Y. & Walsh N.G. 2002. Morphological variation in Leptorhynchos squamatus (Gnaphalieae: Asteraceae). Aus. Syst. Bot. 15: 205–219.
Grabherr G., Gottfried M. & Pauli H. 1994. Climate effects on mountain plants. Nature 369: 448–448.
Graefe S., Leuschner C., Coners H. & Hertel D. 2011. Root functioning in tropical high-elevation forests: Environmental vs. biological control of root water absorption. Environ. Exp. Bot. 71: 329–336.
Griffiths R.P., Madritch M.D. & Swanson A.K. 2009. The effects of topography on forest soil characteristics in the Oregon Cascade Mountains (USA): Implications for the effects of climate change on soil properties. For. Ecol. Manage. 257: 1–7.
Gupta S.M., Grover A. & Ahmed Z. 2012, Identification of Abi-otic Stress Responsive Genes from Indian High Altitude Le-pidium, latifolium L. Defence Sci. J. 62: 315–318.
Hameed M., Ashraf M. & Naz N. 2009. Anatomical adaptations to salinity in cogon grass [Imperata cylindrico, (L.) Raeuschel] from the Salt Range, Pakistan. Plant Soil 322: 229–238.
Hameed M., Ashraf M., Naz N. & Al-Qurainy F. 2010. Anatomical adaptations of Cyanodon dactylon (L.) Pers. from the Salt range Pakistan, to salinity stress. I. Root and Stem anatomy. Pak. J. Bot. 42: 279–289.
Hameed M., Nawaz T., Ashraf M., Naz N., Batool R., Ahmad M.S.A. & Riaz A. 2013. Physioanatomical adaptations in response to salt stress in Sporobolus arabicus (Poaceae) from the Salt Range, Pakistan. Turk. J. Bot. 37: 715–724.
Hameed M., Nawaz T., Ashraf M., Tufail A., Kanwal H., Ahmad M.S.A. & Ahmad I. 2012. Leaf anatomical adaptations of some halophytic and xerophytic sedges of the Punjab. Pak. J. Bot. 44: 159–164.
Horie T., Karahara I. & Katsuhara M. 2012. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 5: 1–18.
Hovenden M.J & Vander Schoor J.K. 2004. Nature versus nurture in the leaf morphology of Southern beech, Nothofagus cunninghamii (Nothofagaceae). New Phytol. 161: 585–594.
Hovenden M.J. & Vander Schoor J.K. 2006. The response of leaf morphology to irradiance depends on altitude of origin in Nothofagus cunninghamii. New Phytol. 169: 291–297.
Jiang F., Wang F., Wu Z., Li Y., Shi G., Hu J. & Hou X. 2011. Components of the Arabidopsis CBF coldresponse pathway are conserved in non-heading chinese cabbage. Plant Mol. Biol. Rep. 29: 525–532.
Jump A.S. & Penuelas J. 2005. Running to stand still: adapta-tion and the response of plants to rapid climate change. Ecol. Letters 8: 1010–1020.
Kofidis G., Bosabalidis A.M. & Moustakas M. 2003. Contemporary seasonal and altitudinal variations of leaf structural features in oregano (Origanum, vulgare L.). Ann. Bot. 92: 635–645.
Kofidis G., Bosabalidis A.M. & Moustakas M. 2007. Com-bined effect of altitude and season on leaf characteristics of Clinopodium vulgare L. (Labiatae). Environ. Exp. Bot. 60: 69–76.
Körner C. 1999. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer.
Körner C., Bannister P. & Mark A.F. 1986. Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in different plant life forms in New Zealand. Oecologia 69: 577–588.
Körner C. & Diemer M. 1994. Evidence that plants from high altitudes retain their greater photosynthetic efHciency under elevated C02. Func. Ecol. 8: 58–68.
Körner C., Neumayer M., Menendez-Riedl S. & Smeets-Scheel A. 1989. Functional morphology of mountain plants. Flora 182: 353–383.
Liu Li., Xu S.M. & Woo K.C. 2005. Solar UV-B radiation on growth, photosynthesis and the xanthophylls cycle in tropical acacias and eucalyptus. Environ. Exp. Bot. 54: 121–130.
Malik N.Z., Arshad M. & Mirza S.N. 2007. Phytosociological Attributes of Different Plant Communities of Pir-Chinasi Hills of Azad Jammu and Kashmir. Int. J. Agri. Biol. 9: 569–574.
Mark A.F., Dickinson K.J.M. & Hofstede R.G.M. 2000. Alpine vegetation, plant distribution, life forms, and environments in a humid New Zealand region: Oceanic and tropical high mountain affinities. Arct. Antarct. Alp. Res. 32: 240–254.
McElwain J.C. 2004. Climate-independent paleoaltimetry using stomatal density in fossil leaves as a proxy for C02 partial pressure. Geology 32: 1017–1020.
Peng Y.H., Zhu Y.F. & Mao Y.Q. 2004. Alkali grass resists salt stress through high K+ and an endodermis barrier to Na+. J. Exp. Bot. 55: 939–949.
Poorter L. & Rozendaal D.M.A. 2008. Leaf size and leaf display of 38 tropical tree species. Oecologia 158: 35–46.
Roderick M.L., Berry S.L. & Noble I.R. 2000. A framework for understanding the relationship between environment and vegetation based on the surface area to volume ratio of leaves. Func. Ecol. 14: 423–437.
Royer D.L. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 114: 1–28.
Rundgren M. 1999. A Holocene CO2 record from the stomatal index of subfossil Salix herbacea L. leaves from northern Swe-den. Holocene 9: 509–513.
Sandve S.R., Kosmala A., Rudi H., Fjellheim S., Rapacz M., Yamada T. & Rognli O.A. 2011. Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates. Plant Sci. 180: 69–77.
Schneider J.V., Zipp D., Gaviria J. & Zizka G. 2003 Successional and mature stands in an upper Andean rain forest transect of Venezuela: do leaf characteristics of woody species differ? J. Trop. Ecol.19: 251–259.
Schreiber L., Hartmann K. & Skrabs M. 1999. Apoplastic barriers in roots: Chemical composition of endodermal and hypodermal cell walls. J. Exp. Bot. 50: 1267–1280.
Schroth G., Lehmann J. and Barrios E. 2003. Soil nutrient availability and acidity. In: Schroth G. & Sinclair F.L. (eds), Trees, Crops and Soil Fertility, CAB International, Wallingford, 2: 104–106.
Taguchi Y. & Wada N. 2001. Variations of leaf traits of an alpine shrub Sieversio, pentapetala along an altitudinal gradient and under a stimulated environmental change. Polar Biosci. 14: 79–87
Tanner E.V. & Kapos V. 1982. Leaf structure of Jamaican upper montane rain-forest trees. Biotropica 14: 16–24.
Turner I.M. 1994. Sclerophylly: primarily protective? Fun. Ecol. 9: 279–284.
Vasellati V., Oesterheld M., Medan D. & Loreti J. 2001. Effects of flooding and drought on the anatomy of Paspalum düatatum. Ann. Bot. 88: 355–360.
Wolf B. 1982. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 13: 1035–1059.
Zhu Z.-J., Zhang Y., Hu Y.-Y. & Yan S.- G. 2000. Studies on microscopic structure of Puccinellio, tenuiflora stem under salinity stress. Grassland China 5: 6–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, K.S., Hameed, M., Deng, J. et al. Ecotypic adaptations in Bermuda grass (Cynodon dactylon) for altitudinal stress tolerance. Biologia 71, 885–895 (2016). https://doi.org/10.1515/biolog-2016-0113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1515/biolog-2016-0113