Abstract
Background
Optimal management of colorectal liver metastasis (CRLM) is based on a combination of chemotherapy and surgical resection. The tumor regression grade (TRG) score is a histological scoring system to evaluate response to chemotherapy. The prognosis of a heterogeneous response in cases of multiple metastases has not been evaluated according to the TRG score.
Patients and Methods
All patients who underwent liver resection for multiple CRLM after neoadjuvant chemotherapy in two tertiary centers from January 2015 to April 2019 were retrospectively included. Oncological characteristics and outcome between TRG 1–2–3 (good response group), TRG 4–5 (poor response group) and heterogeneous TRG (good and poor TRG among different lesions within the same patient) groups were compared.
Results
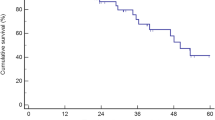
Among the 327 patients included, 134 (41.0%) had good response (TRG 1–2–3), 120 (36.7%) had poor response (TRG 4–5), and 73 (22.3%) had heterogeneous response. The type and number of cycles of chemotherapy, k-Ras mutational status, and tumor number or size did not differ between the three groups. Use of irinotecan-based and anti-VEGF neoadjuvant therapy was associated with better TRG response [irinotecan-based: hazard ratio (OR) = 1.744; p = 0.045; anti-VEGF neoadjuvant therapy: 2.054; p = 0.005). Overall survival (OS) was higher in the 1–2–3 TRG group than in the heterogeneous TRG group (2-year OS = 81.3% vs. 60.3%, respectively; p = 0.003) and the 4–5 TRG group (2-year OS = 81.3% vs. 55.0%, respectively; p = 0.012) and similar between the heterogeneous and 4–5 TRG groups.
Conclusions
The proportion of heterogeneous pathological response according to TRG is 22.3%, and the prognosis is comparable to that of poor pathological response.



Similar content being viewed by others
References
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208–15.
Husband JE, Schwartz LH, Spencer J, Ollivier L, King DM, Johnson R, et al. Evaluation of the response to treatment of solid tumours—a consensus statement of the International Cancer Imaging Society. Br J Cancer. 2004;90(12):2256–60.
Jang HJ, Kim BC, Kim HS, Kim JH, Song HH, Kim JB, et al. Comparison of RECIST 1.0 and RECIST 1.1 on computed tomography in patients with metastatic colorectal cancer. Oncology. 2014;86(2):117–21.
Komatsu S, Scatton O, Goumard C, Sepulveda A, Brustia R, Perdigao F, et al. Development process and technical aspects of laparoscopic hepatectomy: learning curve based on 15 years of experience. J Am Coll Surg. 2017;224(5):841–50.
Wakabayashi G, Cherqui D, Geller DA, Abu Hilal M, Berardi G, Ciria R, et al. The Tokyo 2020 terminology of liver anatomy and resections: updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci. 2022;29(1):6–15.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242(6):824–8 (discussion 8–9).
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6.
Birgin E, Tesfazgi W, Knoth M, Wilhelm TJ, Post S, Ruckert F. Evaluation of the new ISGLS definitions of typical posthepatectomy complications. Scand J Surg. 2019;108(2):130–6.
Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299–304.
Mentha G, Majno PE, Andres A, Rubbia-Brandt L, Morel P, Roth AD. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg. 2006;93(7):872–8.
Delaunoit T, Alberts SR, Sargent DJ, Green E, Goldberg RM, Krook J, et al. Chemotherapy permits resection of metastatic colorectal cancer: experience from intergroup N9741. Ann Oncol. 2005;16(3):425–9.
Mason MC, Krasnodebski M, Hester CA, Kothari AN, Barker C, Nishioka Y, et al. Outcomes of mixed pathologic response in patients with multiple colorectal liver metastases treated with neoadjuvant chemotherapy and liver resection. Ann Surg Oncol. 2022;29(8):5156–64.
Vigano L, Capussotti L, De Rosa G, De Saussure WO, Mentha G, Rubbia-Brandt L. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann Surg. 2013;258(5):731–40 (discussion 41–2).
Poultsides GA, Bao F, Servais EL, Hernandez-Boussard T, DeMatteo RP, Allen PJ, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012;19(9):2797–804.
Sebagh M, Bosselut N, Santos AD, Allard MA, Ruiz A, Saffroy R, et al. Rare genetic heterogeneity within single tumor discovered for the first time in colorectal liver metastases after liver resection. Oncotarget. 2018;9(31):21921–9.
Jacome AA, Oliveira FA, Lino F, Lima J. Effect of adding bevacizumab to chemotherapy on pathologic response to preoperative systemic therapy for resectable colorectal liver metastases: a systematic review and meta-analysis. Clin Colorectal Cancer. 2021;20(3):265–72.
Pietrantonio F, Mazzaferro V, Miceli R, Cotsoglou C, Melotti F, Fanetti G, et al. Pathological response after neoadjuvant bevacizumab- or cetuximab-based chemotherapy in resected colorectal cancer liver metastases. Med Oncol. 2015;32(7):182.
Thonhauser R, Poglitsch M, Jonas JP, Dong Y, Tschogl M, Gramberger M, et al. The effect of induction chemotherapy with VEGF inhibition on tumor response in synchronously metastasized potentially resectable colorectal cancer. Cancers. 2023;15(11):2900.
Blazer DG 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26(33):5344–51.
Sato H, Ii T, Rikiyama N, Noguchi A, Aoki Y, Sato J, et al. Two cases of resectable liver metastasis from colorectal cancer with pathological complete response after neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 2020;47(13):1954–6.
Tabchouri N, Gayet B, Okumura S, Donatelli G, Beaussier M, Bennamoun M, et al. Recurrence patterns after laparoscopic resection of colorectal liver metastases. Surg Endosc. 2018;32(12):4788–97.
Funding
No funding or support was provided. The authors have no conflicts of interest to declare. There has been no previous communication with any society or meeting regarding this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laroche, S., Scatton, O., Charlotte, F. et al. Prognosis of a Heterogeneous TRG Pathological Response to Neoadjuvant Chemotherapy in Patients who Undergo Resection for Colorectal Liver Metastases. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15196-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15196-x