Abstract
Background
Novel definitions suggest that resectability status for pancreatic ductal adenocarcinoma (PDAC) should be assessed beyond anatomical criteria, considering both biological and conditional factors. This has, however, yet to be validated on a nationwide scale. This study evaluated the prognostic value of biological and conditional factors for staging of patients with resectable PDAC.
Patients and Methods
A nationwide observational cohort study was performed, including all consecutive patients who underwent upfront resection of National Comprehensive Cancer Network resectable PDAC in the Netherlands (2014–2019) with complete information on preoperative carbohydrate antigen (CA) 19-9 and Eastern Cooperative Oncology Group (ECOG) performance status. PDAC was considered biologically unfavorable (RB+) if CA19-9 ≥ 500 U/mL and favorable (RB−) otherwise. ECOG ≥ 2 was considered conditionally unfavorable (RC+) and favorable otherwise (RC−). Overall survival (OS) was assessed using Kaplan–Meier and Cox-proportional hazard analysis, presented as hazard ratios (HRs) with 95% confidence interval (CI).
Results
Overall, 688 patients were analyzed with a median overall survival (OS) of 20 months (95% CI 19–23). OS was 14 months (95% CI 10 months—median not reached) in 20 RB+C+ patients (3%; HR 1.61, 95% CI 0.86–2.70), 13 months (95% CI 11–15) in 156 RB+C− patients (23%; HR 1.86, 95% CI 1.50–2.31), and 21 months (95% CI 12–41) in 47 RB−C+ patients (7%; HR 1.14, 95% CI 0.80–1.62) compared with 24 months (95% CI 22–27) in 465 patients with RB−C− PDAC (68%; reference).
Conclusions
Survival after upfront resection of anatomically resectable PDAC is worse in patients with CA19-9 ≥ 500 U/mL, while performance status had no impact. This supports consideration of CA19-9 in preoperative staging of resectable PDAC.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Despite the development of more effective systemic therapies, pancreatic ductal adenocarcinoma (PDAC) remains associated with a 5-year survival of about 10%.1 For patients with localized PDAC, pancreatic resection combined with systemic therapy is considered standard treatment.2,3,4 In contrast to formerly preferred upfront resection followed by adjuvant chemotherapy, neoadjuvant treatment has gained interest over the last decennium. In patients with borderline resectable PDAC, neoadjuvant therapy has been proven to provide survival benefits and has therefore become the recommended treatment strategy in recent years.5,6,7,8 For patients with primary resectable PDAC, however, definitive results of ongoing randomized controlled trials on the role of neoadjuvant treatment are awaited.9,10,11
Most definitions classify primary pancreatic tumors as resectable, borderline resectable or locally advanced on the basis of the degrees of tumor contact with major vessels, vein irregularity, and thrombosis assessed on radiological imaging.12,13,14 Internationally, the most commonly used resectability criteria are the National Comprehensive Cancer Network (NCCN) guidelines, which define pancreatic tumors as resectable in case of no arterial contact and ≤ 180° portomesenteric venous tumor contact without vein contour irregularity.12
Treatment recommendations of current guidelines are generally based on these anatomical criteria only.12,13,14 Nevertheless, biochemical and conditional factors are known to influence the prognosis of PDAC as well.15,16,17 Biological factors include preoperative serum carbohydrate antigen (CA)19-9 and preoperative regional lymph node metastasis, while the patients’ condition is reflected by the Eastern Cooperative Oncology Group (ECOG) performance status.18 Recently, the International Association of Pancreatology (IAP) has proposed to expand the preoperative staging criteria by redefining borderline resectable PDAC with biological and conditional criteria, suggesting that resectability status should be assessed beyond the anatomic relationship between tumor and vessels.19 This has, however, yet to be validated for patients undergoing upfront resection in a nationwide setting.
Therefore, the aim of this study was to evaluate the prognostic value of biological and conditional factors for staging patients with primary resectable pancreatic cancer.
Patients and Methods
Study Design
A nationwide observational cohort study was performed in all 16 Dutch centers for pancreatic cancer surgery. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies were followed.20 Patients who underwent upfront resection of histologically proven, primary resectable PDAC according to the NCCN criteria between 2014 and 2019 were identified from the mandatory Dutch Pancreatic Cancer Audit (DPCA).21 Patients with an unknown resectability status were excluded. During the study period, the recommended treatment strategy for patients with resectable PDAC was upfront resection. Neoadjuvant treatment was only administered in the context of randomized trials.5,9 Therefore, patients who received neoadjuvant treatment were excluded from this study.
Data Collection
Prospective baseline characteristics and perioperative data were retrieved from the audit database after approval of the DPCG scientific committee. Additionally, detailed data on adjuvant therapy, follow-up, and survival were retrieved from patients’ medical records. Data on ethnicity and race of patients were not obtained, as these data are not available in the DPCA.21
For each patient, anatomical resectability status was determined retrospectively according to NCCN criteria.12 Subsequently, patients with primary resectable (R) PDAC were categorized on the basis of biological and conditional factors. Patients were considered to have biologically unfavorable PDAC (RB+) when preoperative serum CA19-9 level was ≥ 500 U/mL. Patients with serum CA19-9 levels < 500 U/mL were considered RB−. Patients were deemed to have conditionally unfavorable PDAC (RC+) if their baseline ECOG performance status was ≥ 2, and patients with an ECOG performance status of 0–1 were classified as having RC− PDAC.17,19 Based on these criteria, patients were stratified into one of four groups, having either (1) RB+C+ PDAC; (2) RB+C− PDAC; (3) RB−C+ PDAC or (4) RB−C− PDAC. The serum CA19-9 level closest to the date of resection was used if multiple preoperative serum CA19-9 samples were available.
Outcomes
The primary outcome was overall survival (OS), defined as the time between the date of tumor resection until the date of death from any cause. The secondary outcome was disease-free survival (DFS), defined as the time between the date of tumor resection until the date of PDAC recurrence diagnosis. PDAC recurrence was pathologically proven, or suspected through imaging, and preferably confirmed by consensus during a multidisciplinary meeting. Alive patients were censored at the date of last follow-up.
Statistical Analysis
Statistical analysis was performed including only patients with complete information on “key variables,” i.e., preoperative serum CA19-9 and ECOG performance status. To assess potential selection bias resulting from the complete case analysis, baseline characteristics of included patients were compared with baseline characteristics of patients who were excluded owing to missing data. Other missing baseline data were considered missing at random and imputed based on a Markov Chain Monte Carlo method (five imputations; ten iterations).22 The original cohort and cohort after multiple imputations were compared for inconsistencies. Descriptive statistics were used to compare the prespecified groups. Categorical variables were presented as frequencies and compared using the chi-squared or Fisher’s exact test. Parametric continuous variables were shown as mean with standard deviation (SD) and compared using one-way analysis of variance (ANOVA). Non-parametric continuous variables were reported as median with interquartile range (IQR) and compared using the Kruskal–Wallis test. Kaplan–Meier survival curves were used to assess OS and DFS for each group, and univariate Cox proportional hazard analyses were performed to calculate survival differences. Results were presented as hazard ratio (HR) with 95% confidence interval (CI).
Several sensitivity analyses were performed to assess robustness of findings when accounting for underlying factors that may affect the results. First, the primary analysis was stratified for presence of hyperbilirubinemia (defined as preoperative total bilirubin serum levels < 20 μmol/L), considering that serum CA19-9 levels may be inaccurate in cases of hyperbilirubinemia.23,24,25 Second, a sensitivity analysis was conducted in a subset of patients with serum CA19-9 levels ≥ 5 U/mL, excluding patients who are considered non or low-secretors of CA19-9.26,27 A third sensitivity analysis was done using a lower CA19-9 threshold for RB+ PDAC, i.e., 200 U/mL, based on the results of a recent study.16 Furthermore, a fourth sensitivity analysis was performed using a lower ECOG threshold, i.e., ≥ 1, to define RC+ PDAC.
Finally, multivariable Cox proportional hazard analyses were conducted to investigate the association between serum CA19-9 and OS, and ECOG performance status and OS, adjusted for potential confounders (i.e., age, sex, tumor size, nodal stage, resection margin status, tumor differentiation, and perineural invasion).
Statistical analyses were performed using R version 3.5.1 (Bell Laboratories, NH, USA) using the “mice” and “survival” packages. A two-tailed P value of ≤ 0.05 was considered statistically significant.
Results
Study Population
Overall, 1906 patients were identified, of whom 1443 underwent upfront resection of NCCN resectable PDAC. Of those, 688 patients were included (Fig. 1). No differences in baseline characteristics were observed in patients who were excluded owing to missing “key variables” compared with included patients (Supplementary Digital Content 1).
Median follow-up was 31 months (IQR 20–46 months), median OS was 20 months (95% CI 19–23 months) and median DFS was 15 months (95% CI 14–17 months). The first sites of recurrence were local (22%), liver (15%), and multiple (54%). Stratification of the cohort resulted in 20 patients (3%) with RB+C+ PDAC, 156 patients (23%) with RB+C− PDAC, 47 patients (7%) with RB−C+ PDAC, and 465 patients (68%) with RB−C− PDAC (Table 1). Groups differed significantly with regard to age ASA III–IV, BMI, CCI score, serum bilirubin levels, vascular resection, hospital stay, pathologically measured tumor size, nodal stage, resection margin status, and adjuvant chemotherapy.
Survival
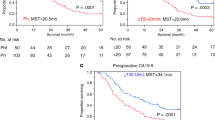
Patients classified as having RB+C+ PDAC had a median OS of 14 months (95% CI 10 months—median not reached). Median OS was 13 months (95% CI 11–15 months) for patients with RB+C– PDAC, 21 months (95% CI 12–41 months) for patients with RB–C+ PDAC, and 24 months (95% CI 22–27 months) for patients with RB–C− PDAC (Fig. 2). Compared with RB–C– PDAC (reference), this resulted in a HR of 1.61 (95% CI 0.86–2.70; P = 0.07) for patients with RB+C+ PDAC, a HR of 1.86 (95% CI 1.50–2.31; P < 0.001) for patients with RB+C− PDAC, and a HR of 1.14 (95% CI 0.80–1.62; P = 0.48) for patients with RB–C+ PDAC.
Kaplan–Meier curves and results from Cox proportional hazard analysis comparing overall survival between patients with RB+C+, RB+C−, RB−C+, and RB−C− PDAC; RB+ was defined as preoperative serum CA19-9 levels ≥ 500 U/mL and RB− as CA19-9 < 500 U/mL; RC+ was considered with an ECOG performance status ≥ 2 and RC− with ECOG 0–1
Sensitivity Analysis
Stratification for presence of hyperbilirubinemia identified 386 patients (56%) with hyperbilirubinemia and 302 patients (44%) without hyperbilirubinemia. In both patients with and without hyperbilirubinemia, the RB+C– group had the lowest survival of all groups, being 13 months (95% CI 11–16 months), and 11 months (95% CI 10–22 months), respectively (Supplementary Digital Content 2). Results remained the same when excluding non-secretors of CA19-9 (n = 44; 6%) (Supplementary Digital Content 3).
A lower serum CA19-9 threshold of ≥ 200 U/mL for defining RB+ resulted in more patients being staged as having RB+C+ PDAC (n = 29, 4%), and RB+C– PDAC (n = 297, 43%). Similar survival differences were found between reclassified groups, with RB+C+ PDAC having the worst median OS of 12 months (95% CI 10–17 months; Fig. 3). A lower threshold for RC+ (ECOG performance status ≥ 1; Supplementary Digital Content 4) resulted in a larger number of patients considered RB+C+ (n = 100; 15%), while the number of patients in the RB–C+ (n = 248; 36%) group also increased. Interestingly, survival was now lowest for patients in the RB+C+ PDAC group, with a median OS of 12 months (95% CI 10–14 months).
Sensitivity analysis: Kaplan–Meier curves and Cox-proportional hazard analysis comparing overall survival between patients with RB+C+, RB+C−, RB−C+, and RB−C− PDAC, defining RB+ as CA19-9 ≥ 200 U/mL; RB+ was defined as preoperative serum CA19-9 levels ≥ 200 U/mL and RB− as CA19-9 < 200 U/mL; RC+ was considered with an ECOG performance status ≥ 2 and RC− with ECOG 0–1
Association Between Biological Factors, Conditional Factors, and OS
Multivariable analysis identified a preoperative CA19-9 serum level ≥ 500 U/mL [HR 1.62 (95% CI 1.31–1.99); P < 0.001] to be associated with OS. A lower CA19-9 threshold of ≥ 200 U/mL showed a similar association [HR 1.53 (95% CI 1.27–1.84); P < 0.001]. Worse ECOG performance status (≥ 2 versus 0–1) was not associated with OS [HR 0.99 (95% CI 0.72–1.33); P = 0.94]. This remained the same when lowering the ECOG threshold [≥ 1 versus 0; HR 1.08 (95% CI 0.90–1.31); P = 0.39].
Discussion
This study showed that survival after upfront resection is significantly worse for patients with anatomically resectable but biologically unfavorable PDAC (serum CA19-9 ≥ 500 U/mL) than for patients with resectable PDAC and serum CA19-9 < 500 U/mL. ECOG performance status did not impact survival. Similar survival differences were found when applying a lower preoperative serum CA19-9 threshold (≥ 200 U/mL), affecting an even larger group of patients. These findings suggest that serum CA19-9 levels are valuable for preoperative staging of patients with resectable PDAC.
The resectability definition for localized PDAC is traditionally based on vascular tumor involvement. This definition was introduced to identify patients with LAPC or borderline resectable disease who have a higher risk of R1 resections, in which initial systemic treatment instead of upfront resection is recommended.28 Currently, most guidelines regarding treatment strategies of PDAC focus on anatomical criteria only, although the value of biological and conditional factors is increasingly emphasized.18,29 Considering that accurate preoperative staging of PDAC patients is relevant for guiding treatment strategies and supporting shared-decision making, evaluation of the additional value of these factors is of great importance.30
Previously, two smaller single-center studies were published to validate the IAP proposal, including both biological and conditional factors.31,32 Hayasaki et al. studied 285 patients who received preoperative neoadjuvant chemoradiotherapy, reflecting a different patient group than patients who underwent upfront resection in our study.32 The study by Kato et al. included only 12 conditionally unfavorable patients, impeding a proper analysis of the value of conditional factors.31 The current study is the first to validate the IAP proposal in a large, nationwide cohort of patients who underwent upfront resection. This resulted in a larger number of patients available for analysis in each subgroup, enhancing the power of findings. Moreover, in contrast to previous studies, this study also investigates the interplay of biological and conditional factors, providing a deeper understanding.
An important finding of this study was that, despite having anatomically resectable PDAC, patients with RB+ disease have a dismal prognosis when compared with patients with RB– disease. After correction for potential inaccuracy of CA19-9 owing to hyperbilirubinemia and after exclusion of non or low-secretors of CA19-9, survival differences remained at a similar disadvantage for the RB+ PDAC group. This supports the importance of tumor biology to stage patients beyond anatomical resectability criteria, as also underlined by recent studies demonstrating lower survival rates in patients with biologically unfavorable PDAC.31,32,33,34,35 Nevertheless, although previous studies showed a strong association between preoperative serum CA 19-9 levels and survival, most international guidelines do not incorporate CA19-9 in the treatment recommendations for localized PDAC.16,23,31,32,33,34,35,36,37 One study determined CA19-9 ≥ 200 U/mL to be associated with worse survival outcomes after PDAC resection, while another study established the threshold of CA19-9 at 1000 U/mL.16,31 Considering that a lower cutoff for CA19-9 may impact a higher number of patients, a sensitivity analysis was performed with a less strict threshold of CA19-9 ≥ 200 U/mL for RB+. Survival outcomes after upfront resection for the larger group of RB+ patients with a serum CA 19-9 ≥ 200 U/mL were significantly worse compared with the other PDAC groups. Therefore, a preoperative serum CA19-9 threshold of ≥ 200 U/mL may be considered for preoperative staging of resectable PDAC.
In contrast to the widely studied importance of preoperative serum CA19-9, less is known about the need to incorporate conditional factors for preoperative staging of resectable PDAC. Conditional factors might be important, as they are negatively associated with complications after surgery, refraining from chemotherapy, and poor survival.17,38 Previous studies reported ECOG performance status to be a major prognostic factor for survival in patients with PDAC.17,31,32,38 Moreover, other factors reflecting conditional status have also been associated with survival, such as radiomics and body composition measures on preoperative imaging.39,40 Nevertheless, only the ECOG performance status was included in proposed staging criteria. In our study, however, survival in patients with RB–C+ PDAC was similar to survival in RB–C– patients. For the small group of patients with RB+C+, survival outcomes seemed mainly disadvantaged because of an unfavorable tumor biology. Furthermore, ECOG performance status was not associated with decreased OS in a multivariable model. Nevertheless, patients with a poor performance status might have been determined eligible for surgery only after careful selection during multidisciplinary team meetings. These meetings have been initiated to screen patients on frailty and surgical risk, herewith improving patient selection while also paying attention to prehabilitation to improve patient fitness before surgery.41 Consequently, failure to demonstrate a difference in survival between RB–C+ patients and RB–C– might be a result of confounding by indication.
The addition of biological and conditional parameters for preoperative staging has been proposed previously and usually contained elevated preoperative serum CA19-9.18,29,42 The IAP consensus statement regarding novel borderline resectability criteria also considered patients with preoperative regional lymph nodes metastasis, diagnosed by positron emission tomography-computed tomography (PET-CT) or nodal biopsy, to have borderline resectable disease.19 The value of preoperative regional lymph node metastasis as an expression of biologically unfavorable PDAC could not be assessed in this study since PET-CT and nodal biopsy were not performed routinely. Interestingly, however, higher rates of positive pathological lymph nodes were found for patients with RB+. Evaluation of the additive value of biopsy or PET-CT to assess preoperative regional lymph node metastasis could therefore be a potential focus of future studies.
Currently, guidelines recommend administration of neoadjuvant chemo(radio)therapy for anatomically borderline resectable PDAC, supported by the results of recent randomized controlled trials.43 These studies showed better OS after neoadjuvant treatment in borderline resectable patients compared with upfront surgery. Since RB+ patients had a dismal prognosis after upfront tumor resection in our study, neoadjuvant treatment with intensive chemotherapeutic regimens, such as FOLFORINOX, could be suggested for this group. This has already been implemented in clinical practice in some large-volume pancreatic expert centers.18 However, to prove that this is the optimal treatment strategy for these patients, evidence from randomized controlled trials must be obtained.
This study has several limitations. First, the study population consisted only of patients who underwent upfront PDAC resection. Patients initially scheduled for pancreatic resection, but who refrained from resection owing to fast progressive disease and health deterioration, were not included. However, the results of this study are still applicable to the vast majority of patients, since approximately 75% of patients scheduled for surgery eventually undergo PDAC resection.5,44,45 Second, a complete case analysis based on completeness of “key variables” was performed to allow for a more accurate evaluation of the different staging categories. As a result, patients with unknown preoperative serum CA19-9 or ECOG performance status were excluded, causing potential selection bias. However, comparison of characteristics between included and excluded patients revealed no difference. Moreover, the RC+ groups were quite small, so nonsignificant differences may be due to insufficient power. Consequently, the findings with regard to the impact of ECOG performance status as a conditional factor should be interpreted with care. Finally, the current study only included patients with resectable PDAC, as the recommended treatment strategy for borderline resectable PDAC now consists of neoadjuvant therapy instead of upfront resection. Nevertheless, it would be valuable to further explore the impact of biological and conditional factors in patients with borderline resectable PDAC and in the context of neoadjuvant treatment as well, as this could aid in refining patient stratification.
In conclusion, this nationwide observational cohort study demonstrated that patients with anatomically resectable but biologically unfavorable PDAC (defined as a preoperative serum CA19-9 level ≥ 500 U/mL) have worse survival than patients with preoperative serum CA19-9 < 500 U/mL, independent of the patients’ performance status. The inclusion of CA19-9 for preoperative staging of patients with resectable PDAC should be considered, although prospective studies will need to determine whether neoadjuvant treatment is beneficial for these patients.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
Conroy T, Hammel P, Hebbar M, et al. Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–24.
Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Dutch Pancreatic Cancer Group. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83-93.
Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763–73.
Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the dutch randomized PREOPANC trial. J Clin Oncol. 2022;40(11):1220–30.
Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–22.
Cloyd JM, Heh V, Pawlik TM, et al. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med. 2020;9(4):1129.
Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21(1):300. Published 2021 Mar 23.
Ghanem I, Lora D, Herradón N, et al. Neoadjuvant chemotherapy with or without radiotherapy versus upfront surgery for resectable pancreatic adenocarcinoma: a meta-analysis of randomized clinical trials [published online ahead of print, 2022 May 14]. ESMO Open. 2022;7(3):100485.
Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49(2):190–4.
National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. [Accessed December 1, 2023]
Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-68.
Federatie Medische Specialisten. Richtlijn Pancreascarcinoom 2019. Available via: https://richtlijnendatabase.nl/richtlijn/pancreascarcinoom/startpagina.html [Accessed August 30, 2023].
Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate Antigen 19–9 Elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg. 2016;223(1):52–65.
Daamen LA, Dorland G, Brada LJH, et al. Preoperative predictors for early and very early disease recurrence in patients undergoing resection of pancreatic ductal adenocarcinoma. HPB (Oxford). 2022;24(4):535–46.
Tas F, Sen F, Odabas H, Kılıc L, Keskın S, Yıldız I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 2013;18(5):839–46.
Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–48.
Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies.
van Rijssen LB, Koerkamp BG, Zwart MJ, et al. Dutch Pancreatic Cancer Group. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch Pancreatic Cancer Audit. HPB (Oxford). 2017 Oct;19(10):919-926.
Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–91.
Hartwig W, Strobel O, Hinz U, et al. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20(7):2188–96. https://doi.org/10.1245/s10434-012-2809-1.
La Greca G, Sofia M, Lombardo R, et al. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: influence of bilirubin and C-reactive protein. World J Gastroenterol. 2012;18(31):4150–5.
Anger F, Lock JF, Klein I, et al. Does concurrent cholestasis alter the prognostic value of preoperatively elevated CA19-9 serum levels in patients with pancreatic head adenocarcinoma? Ann Surg Oncol. 2022. https://doi.org/10.1245/s10434-022-12460-w.
Tempero, Margaret A., et al. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Research 47.20 (1987): 5501-5503.
Scarà S, Bottoni P, Scatena R. CA 19–9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247–60.
Tzeng CW, Fleming JB, Lee JE, et al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol. 2012;19(6):2045–53. https://doi.org/10.1245/s10434-011-2211-4.
The University of Texas MD Anderson Cancer Center. Pancreatic adenocarcinoma. https://www.mdanderson.org/for-physicians/clinical-tools-resources/clinical-practice-algorithms/cancer-treatment-algorithms.html [Accessed March 22, 2022)
Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? Brit Med J. 2009;338:b375.
Kato Y, Yamada S, Tashiro M, et al. Biological and conditional factors should be included when defining criteria for resectability for patients with pancreatic cancer. HPB (Oxford). 2019;21(9):1211–8.
Hayasaki A, Isaji S, Kishiwada M, et al. Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline resectable cancer. Cancers (Basel). 2018;10(3):65.
Lee B, Yoon YS, Kang M, et al. Validation of the anatomical and biological definitions of borderline resectable pancreatic cancer according to the 2017 international consensus for survival and recurrence in patients with pancreatic ductal adenocarcinoma undergoing upfront surgery. Ann Surg Oncol. 2023;30(6):3444–54. https://doi.org/10.1245/s10434-022-13043-5.
Anger F, Döring A, van Dam J, et al. Impact of borderline resectability in pancreatic head cancer on patient survival: biology matters according to the new International Consensus Criteria. Ann Surg Oncol. 2021;28(4):2325–36. https://doi.org/10.1245/s10434-020-09100-6.
Medrano J, Garnier J, Ewald J, et al. Patient outcome according to the 2017 international consensus on the definition of borderline resectable pancreatic ductal adenocarcinoma. Pancreatology. 2020;20(2):223–8.
Humphris JL, Chang DK, Johns AL, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23(7):1713-1722.
Takahashi H, Yamada D, Asukai K, et al. Clinical implications of the serum CA19-9 level in “biological borderline resectability” and “biological downstaging” in the setting of preoperative chemoradiation therapy for pancreatic cancer. Pancreatology. 2020;20(5):919–28.
Tzeng CW, Katz MH, Fleming JB, et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18(1):146–56.
Shi H, Wei Y, Cheng S, et al. Survival prediction after upfront surgery in patients with pancreatic ductal adenocarcinoma: radiomic, clinic-pathologic and body composition analysis. Pancreatology. 2021;21(4):731–7.
van der Kroft G, Wee L, Rensen SS, et al. Identifying radiomics signatures in body composition imaging for the prediction of outcome following pancreatic cancer resection. Front Oncol. 2023;13:1062937. Published 2023 Aug 10.
Henry AC, Schouten TJ, Daamen LA, et al. Short- and long-term outcomes of pancreatic cancer resection for elderly patients: a nationwide analysis. Ann Surg Oncol. 2022;29(9):6031–42. https://doi.org/10.1245/s10434-022-11831-7.
Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(21):2541–56.
van Dam JL, Janssen QP, Besselink MG, et al. Neoadjuvant therapy or upfront surgery for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomised controlled trials. Eur J Cancer. 2022;160:140–9.
Allen VB, Gurusamy KS, Takwoingi Y, et al. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2016;7:CD009323
Ta R, O’Connor DB, Sulistijo A, et al. The role of staging laparoscopy in resectable and borderline resectable pancreatic cancer: a systematic review and meta-analysis. Dig Surg. 2019;36:251–60.
Funding
This research was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schouten, T.J., van Goor, I.W.J.M., Dorland, G.A. et al. The Value of Biological and Conditional Factors for Staging of Patients with Resectable Pancreatic Cancer Undergoing Upfront Resection: A Nationwide Analysis. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15070-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15070-w