Abstract
Background
Sentinel lymph node (SLN) biopsy for cN+ breast cancer patients after neoadjuvant chemotherapy (NAC) is controversial because the false-negative rate (FNR) is high. Identification of three or more SLNs with a dual tracer improves these results, and inclusion of a clipped lymph node (CLN) (targeted axillary dissection [TAD]) may be even more effective.
Methods
A retrospective, single-institution analysis of consecutive cN+ patients undergoing NAC from 2019 to 2021 was performed. Patients routinely underwent placement of a clip in the positive lymph node before NAC, and TAD was performed after completion of therapy.
Results
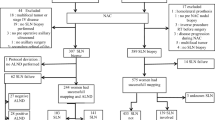
The study analyzed 73 patients, and the identification rate for CLN was 98.6% (72/73). A complete response in the lymph nodes was achieved for 43 (59%) of the 73 patients. Overall, the CLN was not a SLN in 18 (25%) of 73 cases, and for women who had one or two and those who had three or more SLNs identified, this occurred in 11 (32%) and 7 (21%) of 34 cases, respectively. Failure of SLN or TAD to identify a positive residual lymph node status after NAC occurred in 10 (15%) of 69 and 2 (3%) of 73 cases, respectively (p = 0.01). In four cases, a SLN was not retrieved (5.5%), and two of these cases had a positive CLN. In three cases, the CLN was the only positive node and did not match with a SLN, directing lymphadenectomy and oncologic management change in two cases. Therefore, 7 (10%) of 73 cases had a change in surgical or oncologic management with TAD.
Conclusions
For a conservative axillary treatment in this setting, TAD is an effective method. It is more accurate than SLN alone and allows management changes. Further studies are warranted.

Similar content being viewed by others
References
He PS, Li F, Li GH, Guo C, Chen TJ. The combination of blue dye and radioisotope versus radioisotope alone during sentinel lymph node biopsy for breast cancer: a systematic review. BMC Cancer. 2016;16:107.
Shirzadi A, Mahmoodzadeh H, Qorbani M. Assessment of sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer in two subgroups: initially node-negative and node-positive converted to node-negative: a systemic review and meta-analysis. J Res Med Sci. 2019;24:18.
Park S, Park JM, Cho JM, Park HS, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytological proven node-positive breast cancer diagnosis. Ann Surg Oncol. 2013;20:2858–65.
Hiecken TJ, Boughey JC, Jones KN, Shah SS, et al. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol. 2013;20:3199–204.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61.
Mamtani A, Barrio AV, King TA, Van Zee KJ. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastasis? Results of a prospective study. Ann Surg Oncol. 2016;23:3467–74.
Alvarado R, Yi M, le-Petross H, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012;19:3177–84.
Gentile LF, Plitas G, Zabor EC, Stempel M, et al. Tumor biology predicts pathologic complete response to neoadjuvant chemotherapy in patients presenting with local advanced breast cancer. Ann Surg Oncol. 2017;24:3896–902.
Cerbelli B, Botticelli A, Pisano A, Campagna D, et al. Breast cancer subtypes affect the nodal response after neoadjuvant chemotherapy in locally advanced breast cancer: are we ready to endorse axillary conservation? Breast J. 2019;25:273–7. https://doi.org/10.1111/tbj.13206.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: finding from ACOSOG Z1071 (Alliance) prospective multicentric trial. Ann Surg. 2014;260:608–16.
Samiei S, Simons JM, Engelen ME, Beets-Tan RGH, et al. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtypes in patients with initially node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021;156:e210891.
Piltin MA, Hoskin TL, Day CN, Davis J Jr, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27:4795–801. https://doi.org/10.1245/s10434-020-08900-0.
Symmans WF, You C, Chen Y-Y, Balassanian R, et al. Assessment of residual cancer burden and event-free survival in neoadjuvant treatment for high-risk breast cancer: an analysis of data from the I-SPY2 randomized clinical trial. JAMA Oncol. 2021;7:1654–63.
Wong SM, Basik M, Florianova L, et al. Oncologic safety of sentinel lymph node biopsy alone after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2021;28:2621–9. https://doi.org/10.1245/s10434-020-09211-0.
Gerber B, Schneeweiss A, Mobus V, et al. Pathological response in the breast and axillary lymph nodes after neoadjuvant systemic treatment in patients with initially node-positive breast cancer correlates with disease free survival: an exploratory analysis of the GeparOcto trial. Cancers. 2022;14:521.
Kim R, Chang JM, Lee HB, Lee SH, Kim SY, Kim ES, Cho N, Moon WK. Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node-positive breast cancer. Radiology. 2019;293:49–57. https://doi.org/10.1148/radiol.2019190014.
Gradishar WJ, Moran MS, Abraham J et al. Breast Cancer, version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:691–722. https://doi.org/10.6004/jnccn.2022.0030.
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg. 2016;263:802–7.
Vázquez JC, Piñero A, de Castro FJ, et al. The value of sentinel lymph node biopsy in women with node-positive breast cancer at diagnosis and node-negative tumour after neoadjuvant therapy: a systematic review. Clin Transl Oncol. 2023;25:417–28. https://doi.org/10.1007/s12094-022-02953-1.
Gasparri ML, de Boniface J, Poortmans P, et al. Axillary surgery after neoadjuvant therapy in initially node-positive breast cancer: international EUBREAST survey. Br J Surg. 2022;109:857–63. https://doi.org/10.1093/bjs/znac217.
Armer JM, Ballman KV, McCall L, et al. Factors associated with lymphedema in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary dissection. JAMA Surg. 2019;154:800–9. https://doi.org/10.1001/jamasurg.2019.1742.
Straver M, Loo C, Alderliesten T, Rutgers E, Peeters M. Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg. 2010;97:1226–31.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18.
Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers Basel. 2021;13:1539. https://doi.org/10.3390/cancers13071539.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg. 2016;263:802–7. https://doi.org/10.1097/SLA.0000000000001375.
Cabıoğlu N, Karanlık H, Kangal D, Özkurt E, Öner G, Sezen F, et al. Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25:3030–6.
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8.
Siso C, de Torres J, Esgueva-Colmenarejo A, Espinosa-Bravo M, Rus N, Cordoba O, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25:784–91.
Flores-Funes D, Aguilar-Jiménez J, Martínez-Gálvez M, Ibáñez-Ibáñez MJ, Carrasco-González L, Gil-Izquierdo JI, et al. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg Oncol. 2019;30:52–7.
Kuemmel S, Heil J, Rueland A, Seiberling C, Harrach H, Schindowski D, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2020;276(5):e553–62.
Weiss A, King C, Grossmith S, Portnow L, Raza S, Nakhlis F, et al. How often does retrieval of a clipped lymph node change adjuvant therapy recommendations? A prospective, consecutive, patient cohort study. Ann Surg Oncol. 2022;29:3764–71. https://doi.org/10.1245/s10434-022-11324-7.
Cabıoğlu N, Karanlık H, Yıldırım N, Müslümanoğlu M, Çakmak Karadeniz G, Trabulus Can D, et al. Favorable outcome with sentinel lymph node biopsy alone after neoadjuvant chemotherapy in clinically node-positive breast cancer at diagnosis: Turkish Multicentric NEOSENTI-TURK MF-18-02-study. Eur J Surg Oncol. 2021;47:2506–14.
Montagna G, Lee MK, Sivilimedu V, Barrio AV, Morrow M. Is nodal clipping beneficial for node-positive breast cancer receiving neoadjuvant chemotherapy? Ann Surg Oncol. 2022;29:6133–9.
Stankowski-Drengler TJ, Neuman HB. Management of the axilla after neoadjuvant systemic therapy. Curr Treat Options Oncol. 2020;27(21):54.
Biganzoli L, Cardoso F, Beishon M, et al. The requirements of a specialist breast centre. Breast. 2020;51:65–84. https://doi.org/10.1016/j.breast.2020.02.003.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). EJC. 2009;45:228–47.
Hamy A-S, Darrigues L, Laas E, et al. Prognostic value of the residual cancer burden index according to breast cancer subtype: validation on a cohort of BC patients treated by neoadjuvant chemotherapy. PloS One. 2020;15:e0234191. https://doi.org/10.1371/journal.pone.0234191.
https://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3.
Tinterri C, Canavese G, Bruzzi P, Dozin B. NEONOD 2: rationale and design of a multicenter non-inferiority trial to assess the effect of axillary surgery omission on the outcome of breast cancer patients presenting only micrometastasis in the sentinel lymph node after neoadjuvant chemotherapy. Contemp Clin Trials Commun. 2020;17:100496. https://doi.org/10.1016/j.conctc.2019.100496.
Banys-Paluchowski M, Gasparri ML, de Boniface J, et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA study. Cancers. 2021;13:1565.
Curigliano G, Burstein HJ, Winer EP, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2017;28:1700–12. https://doi.org/10.1093/annonc/mdx308.
Naffouje S, Sabesas A, Hoover S, et al. Surgical management of the axilla of HER2+ breast cancer in the Z1071 era: a propensity-score-matched analysis of the NCDB. Ann Surg Oncol. 2021;28:8777–88.
Naffouje S, Barker V, Lee C, et al. Surgical management of axilla of triple-negative breast cancer in the Z1071 era: a propensity score-matched analysis of the National Cancer Database. Ann Surg Oncol. 2022;29:2985–97.
Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27 [published correction appears in J Clin Oncol. 2008;26:2793]. J Clin Oncol. 2008;26:778–85. https://doi.org/10.1200/JCO.2007.15.0235.7.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis [published correction appears in Lancet. 2019;393:986]. Lancet. 2014;384:164–72]. https://doi.org/10.1016/S0140-6736(13)62422-8.8.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
James M, Dixit A, Robinson B, et al. Outcomes for patients with non-metastatic triple-negative breast cancer in New Zealand. Clin Oncol (R Coll Radiol). 2019;31:17–24. https://doi.org/10.1016/j.clon.2018.09.006.
Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25:784–91.
Lim GH, Gudi M, Teo SY, Ng RP, Yan Z, Lee YS, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy? Oncologist. 2020;25:e1621–7.
Donker M, Straver M, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261:378–82.
Simons J, van Nijnatten T, van der Pol C, et al. Diagnostic accuracy of de-escalated surgical procedure in axilla for node-positive breast cancer patients treated with neoadjuvant systemic therapy: a systematic review and meta-analysis. JAMA Surg. 2022;157:991–9.
Simons JM, van Pelt M, Marinelli A, et al. Excision of both pretreatment marked positive nodes and sentinel nodes improves axillary staging after neoadjuvant systemic therapy in breast cancer. Br J Surg. 2019;106(12):1632–9.
Song Y, Xu Z, Liang MX, et al. Diagnostic accuracy of de-escalated surgical procedure in axilla for node-positive breast cancer patients treated with neoadjuvant systemic therapy: a systematic review and meta-analysis. Cancer Med. 2022;11:4085–103.
Reitsamer R, Peintinger F, Forsthuber E, Sir A. The applicability of Magseed® for targeted axillary dissection in breast cancer patients treated with neoadjuvant chemotherapy. Breast. 2021;57:113–7.
Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016;23(1549–53):18.
Henke G, Knauer B, Ribi K, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018;19:667. https://doi.org/10.1186/s13063-018-3021-9.
Galimberti V, Kahler Ribeiro Fontana S, Vicini E, Morigi C, et al. This house believes that sentinel node biopsy alone is better than TAD after NACT for cN+ patients. Breast. 2023;67:21–5. https://doi.org/10.1016/j.breast.2022.12.010.
Barrio AV, Montagna G, Mamtani A, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy: a rare event. JAMA Oncol. 2021;7:1851–5. https://doi.org/10.1001/jamaoncol.2021.4394.
Martelli G, Barretta F, Miceli R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long-term results of a prospective interventional study. Ann Surg. 2022;276:e544–52. https://doi.org/10.1097/sla.0000000000004562.
Acknowledgments
This work was supported by Fondazione Prometeus, ONLUS, for the development of research and training in oncology. The authors thank Marcia Jane Adams for English assistance in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costarelli, L., Arienzo, F., Broglia, L. et al. Clipping a Positive Lymph Node Improves Accuracy of Nodal Staging After Neoadjuvant Chemotherapy for Breast Cancer Patients, but Does It Drive Management Changes?. Ann Surg Oncol 31, 3186–3193 (2024). https://doi.org/10.1245/s10434-024-15052-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15052-y