Abstract
Background
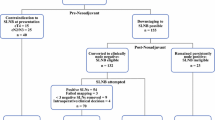
In cN1 patients rendered cN0 with neoadjuvant chemotherapy, the false-negative rate of sentinel lymph node biopsy (SLNB) is < 10% when ≥ 3 sentinel lymph nodes (SLNs) are removed. The added value of nodal clipping in this scenario is unknown. Here we determine how often the clipped node is a sentinel node when ≥ 3 SLNs are retrieved.
Methods
We identified cT1-3N1 patients treated between 02/2018 and 10/2021 with a clipped lymph node at presentation. SLNB was performed with a standardized approach of dual-tracer mapping and retrieval of ≥ 3 SLNs. Clipped nodes were not localized; SLNs were X-rayed intraoperatively to determine clip location. Axillary lymph node dissection (ALND) was performed for any residual disease or retrieval of < 3 SLNs.
Results
Of 269 patients, 251 (93%) had ≥ 3 SLNs. Median age was 51 years; the majority (92%) had ductal histology; 46% were HR+/HER2−. The median number of SLNs removed was 4 (IQR 3,5). The clipped node was an SLN in 88% (220/251) of cases. Of the 31 where the clipped node was not, 13 had a positive SLN mandating ALND, and the clip was identified in the ALND specimen. In the remaining 18, where ≥ 3 negative SLNs were retrieved and an ALND was not performed, the clip was not retrieved, with no axillary failures in this group (median follow-up: 55 months).
Conclusion
When the SLNB procedure is optimized with dual tracer and retrieval of ≥ 3 SLNs, the clipped node is an SLN in the majority of cases, suggesting that failure to retrieve the clipped node should not be an indication for ALND.


Similar content being viewed by others
References
Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21.
Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. 2020;396(10257):1090–100.
Samiei S, Simons JM, Engelen SME, et al. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021;156(6):e210891.
Mamtani A, Barrio AV, King TA, et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23(11):3467–74.
Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol. 2020;27(11):4515–22.
Simons JM, Koppert LB, Luiten EJT, et al. De-escalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. 2020;180(3):725–33.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Classe JM, Loaec C, Gimbergues P, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173(2):343–52.
Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105(12):1541–52.
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–82.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (alliance). Ann Surg. 2016;263(4):802–7.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34(10):1072–8.
Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25(3):784–91.
Diego EJ, McAuliffe PF, Soran A, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016;23(5):1549–53.
Kuemmel S, Heil J, Rueland A, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg. 2020.
Cabioglu N, Karanlik H, Kangal D, et al. Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(10):3030–6.
Simons JM, Nijnatten TJA, Koppert LB, et al. Abstract No. GS1-10. Radioactive iodine seed placement in the axilla with the sentinel lymoh node biopsy after neoadjuvant chemotherapy in breast cancer: results of the prospective multicenter RISAS trial. Cancer Res. 2021;81(4_Supplement):GS1-10.
Simons JM, Maaskant-Braat AJG, Luiten EJT, et al. Patterns of axillary staging and management in clinically node positive breast cancer patients treated with neoadjuvant systemic therapy: results of a survey amongst breast cancer specialists. Eur J Surg Oncol. 2020;46(1):53–8.
Caudle AS, Bedrosian I, Milton DR, et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of american society of breast surgeons members. Ann Surg Oncol. 2017;24(10):2925–34.
Banys-Paluchowski M, Gasparri ML, de Boniface J, et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA Study. Cancers (Basel). 2021;13(7):1565.
Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47(4):804–12.
Wong SM, Basik M, Florianova L, et al. Oncologic safety of sentinel lymph node biopsy alone after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2021;28(5):2621–9.
Martelli G, Barretta F, Miceli R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long-term results of a prospective interventional study. Ann Surg. 2020.
Piltin MA, Hoskin TL, Day CN, Davis J Jr, Boughey JC. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol. 2020;27(12):4795–801.
Barrio AV, Montagna G, Mamtani A, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy: a rare event. JAMA Oncol. 2021;7(12):1851–5.
Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44(9):1307–11.
Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol. 2017;24(10):3011–6.
Wu S, Wang Y, Zhang N, et al. Intraoperative touch imprint cytology in targeted axillary dissection after neoadjuvant chemotherapy for breast cancer patients with initial axillary metastasis. Ann Surg Oncol. 2018;25(11):3150–7.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–59.
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–28.
Weiss A, King C, Grossmith S, et al. How often does retrieval of a clipped lymph node change adjuvant therapy recommendations? A prospective, consecutive, patient cohort study. Ann Surg Oncol. 2022;29:3764–71.
Hartmann S, Stachs A, Schultek G, Gerber B, Reimer T. The clinical relevance of target lymph node biopsy after primary systemic therapy in initially node-positive breast cancer patients. Cancers (Basel). 2021;13(11):2620.
Boughey JC, Ballman KV, McCall LM, et al. Tumor biology and response to chemotherapy impact breast cancer-specific survival in node-positive breast cancer patients treated with neoadjuvant chemotherapy: long-term follow-up from ACOSOG Z1071 (alliance). Ann Surg. 2017;266(4):667–76.
Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017;152(7):665–70.
van der Noordaa MEM, van Duijnhoven FH, Cuijpers FNE, et al. Toward omitting sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with clinically node-negative breast cancer. Br J Surg. 2021;108(6):667–74.
de Wild SR, Simons JM, VranckenPeeters M, Smidt ML, Koppert LB, Group M. MINImal vs. MAXimal invasive axillary staging and treatment after neoadjuvant systemic therapy in node positive breast cancer: protocol of a Dutch multicenter registry study (MINIMAX). Clin Breast Cancer. 2022;22(1):e59–64.
Oncoplastic Breast Consortium (OPBC). Nodal recurrence following axillary downstaging with neoadjuvant chemotherapy and omission of axillary lymph node dissection (OPBC-04/EUBREAST-06). https://oncoplasticbc.org/documents/research/synopsis_opbc04_eubreast06_20211208.pdf Accessed 31 March 2022 (2021).
Acknowledgement
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Monica Morrow has received honoraria from Exact Sciences and Roche. All other authors have no conflict of interest disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was a podium/oral presentation at the 23rd Annual Meeting of the American Society of Breast Surgeons, April 6-10, 2022, Las Vegas, NV.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Montagna, G., Lee, M.K., Sevilimedu, V. et al. Is Nodal Clipping Beneficial for Node-Positive Breast Cancer Patients Receiving Neoadjuvant Chemotherapy?. Ann Surg Oncol 29, 6133–6139 (2022). https://doi.org/10.1245/s10434-022-12240-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12240-6