Abstract
Background
Although various biomarkers are useful in predicting cancer prognosis, the most effective preoperative systemic biomarkers for pancreatic ductal adenocarcinoma (PDAC) have not been established. This study aimed to evaluate whether the lymphocyte-to-monocyte ratio (LMR) can predict the long-term outcomes for patients who were to undergo surgical resection of PDAC.
Methods
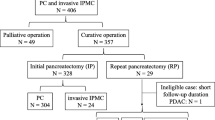
The study involved 170 patients with PDAC who underwent resection. Multivariate analysis was performed to identify the independent prognostic factors for overall survival (OS) and disease-free survival (DFS) among clinicopathologic, surgical, and seven systemic biomarker-related factors including LMR. Subgroup analysis of PDAC located in the body and tail of the pancreas (B/T PDAC) was performed (n = 60) to eliminate the influence of preoperative cholangitis and surgical procedure. Furthermore, OS according to the postoperative course of the LMR value group was investigated.
Results
A low LMR (<3.3) was the only independent predictive factor for OS (hazard ratio [HR], 3.52; p < 0.001) and DFS (HR, 3.31; p < 0.001) among the systemic biomarkers. Subgroup analysis of the B/T PDAC also showed that low the LMR was the independent predictive factor for OS (HR, 3.24; p = 0.002) and DFS (HR, 4.42; p = 0.003). The PDAC that maintained a high LMR from before surgery to 1 year after surgery showed good long-term outcomes (median OS, 8.5 years; 5-year survival rate, 61.8 %).
Conclusions
Preoperative LMR was an independent predictor of OS and DFS after surgery for PDAC. Maintaining a high LMR through the pre- and postoperative courses might improve the prognosis for patients with PDAC.


Similar content being viewed by others
References
Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA. “Artery-first” approaches to pancreatoduodenectomy. Br J Surg. 2012;99:1027–35.
Negoi I, Hostiuc S, Runcanu A, Negoi RI, Beuran M. Superior mesenteric artery first approach versus standard pancreaticoduodenectomy: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2017;16:127–38.
van Roessel S, van Veldhuisen E, Klompmaker S, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant folfirinox treatment. JAMA Oncol. 2020;6:1–8.
Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190–4.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–81.
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-503.
Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015:983698.
Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47.
Zhang LN, Xiao W, Yang PY, et al. The prognostic impact of preoperative blood monocyte count in pathological T3N0M0 rectal cancer without neoadjuvant chemoradiotherapy. Tumour Biol. 2015;36:8213–9.
Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–69.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48:155–70.
Monreal M, Prandoni P. Venous thromboembolism as first manifestation of cancer. Semin Thromb Hemost. 1999;25:131–6.
Edwards CM, Warren J, Armstrong L, Donnelly PK. D-dimer: a useful marker of disease stage in surgery for colorectal cancer. Br J Surg. 1993;80:1404–5.
Hong T, Shen D, Chen X, Wu X, Hua D. Preoperative plasma fibrinogen, but not D-dimer might represent a prognostic factor in non-metastatic colorectal cancer: a prospective cohort study. Cancer Biomark. 2017;19:103–11.
Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44:1494–503.
Watanabe J, Otani S, Sakamoto T, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. 2016;46:1258–67.
Sierzega M, Lenart M, Rutkowska M, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017;24:808–15.
Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–15.
Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085–92.
Yu J, Ding Z, Yang Y, Liu S. Increased platelet-to-lymphocytes ratio is associated with poor long-term prognosis in patients with pancreatic cancer after surgery. Med Baltimore. 2018;97:e11002.
Fang L, Yan FH, Liu C, et al. Systemic inflammatory biomarkers, especially fibrinogen-to-albumin ratio, predict prognosis in patients with pancreatic cancer. Cancer Res Treat. 2021;53:131–9.
Fan N, Chen D, Zheng J, Wen Z, Lin P. A novel preoperative plasma indicator to predict prognoses for patients with esophageal squamous cell carcinoma after radical esophagectomy: fibrinogen-to-lymphocyte ratio. Cancer Manag Res. 2019;11:4719–28.
Wakatsuki K, Matsumoto S, Migita K, et al. Prognostic value of the fibrinogen-to-platelet ratio as an inflammatory and coagulative index in patients with gastric cancer. Surg Today. 2019;49:334–42.
Zang Y, Fan Y, Gao Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: a meta-analysis. Med Baltimore. 2020;99:e20595.
Yasukawa K, Shimizu A, Motoyama H, et al. Preoperative C-reactive protein-to-albumin ratio predicts long-term outcomes in extrahepatic cholangiocarcinoma patients. J Surg Oncol. 2020;122:1094–105.
Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115:470–9.
Lin S, Fang Y, Mo Z, Lin Y, Ji C, Jian Z. Prognostic value of lymphocyte-to-monocyte ratio in pancreatic cancer: a systematic review and meta-analysis including 3338 patients. World J Surg Oncol. 2020;18:186.
Shimizu T, Taniguchi K, Asakuma M, et al. Lymphocyte-to-monocyte ratio and prognostic nutritional index predict poor prognosis in patients on chemotherapy for unresectable pancreatic cancer. Anticancer Res. 2019;39:2169–76.
Tan Z, Zhang M, Han Q, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the fibrinogen/albumin ratio. J Cancer. 2017;8:1025–9.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AI, editors. AJCC Cancer Staging Manual. 7th edn. New York: Springer; 2010.
Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–62.
Tanaka R, Kimura K, Eguchi S, et al. Preoperative neutrophil-to-lymphocyte ratio predicts tumor-infiltrating CD8(+) T cells in biliary tract cancer. Anticancer Res. 2020;40:2881–7.
Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78.
Salmaninejad A, Valilou SF, Soltani A, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol Dordr. 2019;42:591–608.
Suarez-Lopez L, Sriram G, Kong YW, et al. MK2 contributes to tumor progression by promoting M2 macrophage polarization and tumor angiogenesis. Proc Natl Acad Sci U S A. 2018;115:E4236–44.
Boulay BR, Parepally M. Managing malignant biliary obstruction in pancreas cancer: choosing the appropriate strategy. World J Gastroenterol. 2014;20:9345–53.
Li M, Spakowicz D, Burkart J, et al. Change in neutrophil-to-lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145:2541–6.
Jin F, Han A, Shi F, Kong L, Yu J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Onco Targets Ther. 2016;9:6529–37.
Song Q, Wu JZ, Jiang HF, Wang S, Cai SN. The postoperative lymphocyte-to-monocyte ratio change predicts poor clinical outcome in patients with esophageal squamous cell carcinoma undergoing curative resection. Dis Markers. 2020;2020:1451864.
Winters JO, Leider ZL. The value of instant nutritional assessment in predicting postoperative complications and death in gastrointestinal surgical patients. Am Surg. 1983;49:533–5.
Pettigrew RA, Hill GL. Indicators of surgical risk and clinical judgement. Br J Surg. 1986;73:47–51.
Acknowledgment
The authors thank Enago (www.enago.jp), for the English language review. This study was not preregistered in an independent institutional registry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kubota, K., Shimizu, A., Notake, T. et al. Preoperative Peripheral Blood Lymphocyte-to-Monocyte Ratio Predicts Long-Term Outcome for Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 29, 1437–1448 (2022). https://doi.org/10.1245/s10434-021-10848-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10848-8