Abstract
Background
An appropriate strategy is needed to determine the therapeutic effect of chemotherapy on primary lesions in esophageal cancer. This multicenter cohort study aimed to examine the usefulness of a novel criterion obtained by multiplying the lengths of the major and minor esophageal axes from helical computed tomography as a tool to evaluate the therapeutic effect of neoadjuvant chemotherapy and to predict prognosis after surgery in locally advanced esophageal cancer.
Materials and Methods
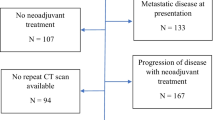
A first investigation evaluated the reproducibility of the new criterion between two independent examiners. In a second investigation, we examined the association of the novel criterion with pathological tumor regression grade and long-term outcomes. Pretreatment primary lesions less than 20 mm on computed tomography were excluded.
Results
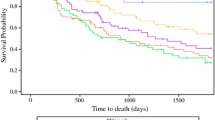
In an initial cohort of 81 patients, the intraclass correlation coefficient for the novel criterion was higher than that for the tumor major axis both before and after neoadjuvant chemotherapy. In the second cohort of 255 patients, the novel criterion significantly correlated with tumor regression grade (p = 0.0003), overall survival (p < 0.0001), and disease-free survival (p < 0.0001). It was also an independent predictor for overall survival (p = 0.0023), along with age, tumor regression grade, and pathological stage.
Conclusions
The measurement derived by multiplying the esophageal major and minor axes on computed tomography is easy to obtain and has better objectivity and reproducibility for tumors of any shape. This novel criterion may be clinically useful because it can estimate therapeutic effect, tumor regression grade, and prognosis after neoadjuvant chemotherapy followed by surgery for esophageal cancer.



Similar content being viewed by others
References
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 2. Esophagus. 2019;16:25–43.
Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:50–7.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–83.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
He Y, Zhao Q, Geng YN, et al. Analysis of short-term efficacy as defined by RECIST and pathological response of neoadjuvant chemotherapy comprised paclitaxel and cisplatin followed by radical surgery in patients with locally advanced cervical cancer: a prospective observational study. Medicine (Baltimore). 2018;97:10913.
Berardi G, De Man M, Laurent S, et al. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur J Surg Oncol. 2018;44:1069–77.
Japan Esophageal Society. Japanese classification of Esophageal cancer, Eleventh Edition: part II and III. Esophagus. 2017;14:37–65.
Sobin LH, Gospodarowicz MK, Wittekind C. International Union against Cancer. TNM classification of malignant tumours. Seventh Edition. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell;2010.
Japan Esophageal Society. Japanese classification of Esophageal Cancer, Eleventh Edition: part I. Jpn Esophageal Soc Esophagus. 2017;14:1–36.
Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Li QW, Qiu B, Wang B, et al. Prediction of pathologic responders to neoadjuvant chemoradiotherapy by diffusion-weighted magnetic resonance imaging in locally advanced esophageal squamous cell carcinoma: a prospective study. Dis Esophagus. 2018. https://doi.org/10.1093/dote/dox121.
Heethuis SE, van Rossum PS, Lips IM, et al. Dynamic contrast-enhanced MRI for treatment response assessment in patients with oesophageal cancer receiving neoadjuvant chemoradiotherapy. Radiother Oncol. 2016;120:128–35.
Izumi D, Yoshida N, Watanabe M, et al. Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2016;51:788–95.
Barbour AP, Walpole ET, Mai GT, et al. Preoperative cisplatin, fluorouracil, and docetaxel with or without radiotherapy after poor early response to cisplatin and fluorouracil for resectable oesophageal adenocarcinoma (AGITG DOCTOR): results from a multicentre, randomised controlled phase II trial. Ann Oncol. 2020;31:236–45.
Domachevsky L, Kashtan H, Brenner B, et al. Baseline 18F-FDG PET/CT as predictor of the pathological response to neoadjuvant therapy in esophageal cancer. Medicine (Baltimore). 2018;97:e13412.
Sonoda A, Yoshida N, Shiraishi S, et al. Total lesion glycolysis ratio in positron emission tomography/computed tomography images during neoadjuvant chemotherapy can predict pathological tumor regression grade and prognosis in patients with locally advanced squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2021;28:167–74.
Fujishima F, Taniyama Y, Nakamura Y, et al. Residual carcinoma cells after chemoradiotherapy for esophageal squamous cell carcinoma patients: striving toward appropriate judgment of biopsy. Dis Esophagus. 2018. https://doi.org/10.1093/dote/dox141.
Chao YK, Wen YW, Chang HK, et al. An analysis of factors affecting the accuracy of endoscopic biopsy after neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43:2366–73.
Eyck BM, Onstenk BD, Noordman BJ, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer: a systematic review and meta-analysis. Ann Surg. 2020;271:245–56.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Acknowledgement
The authors sincerely appreciate Yuichiro Doki, MD, Ph.D., and Koji Tanaka, MD, Ph.D., for their valuable assistance and for providing direction in the conduct of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Naoya Yoshida is affiliated to a department supported by Chugai Pharmaceutical Co., Ltd., and Yakuruto Honsya Co., Ltd., but declares no conflicts of interest regarding this research. The rest of the authors have no conflicts of interest or financial ties to disclose regarding this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshida, N., Taniyama, Y., Murakami, K. et al. Novel Criterion Using Esophageal Major and Minor Axes is Useful to Evaluate the Therapeutic Effect and Prognosis After Neoadjuvant Chemotherapy Followed by Surgery in Locally Advanced Esophageal Cancer. Ann Surg Oncol 28, 8474–8482 (2021). https://doi.org/10.1245/s10434-021-10314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10314-5