Abstract
Background
Serious concerns regarding quality of conduct and reporting of noninferiority trials (NITs) have been raised. Systematic analysis of the quality of the surgical NITs is lacking. Assessing the quality of conduct, reporting, and interpretation of surgical NITs in cancer patients is critical given their potential clinical impact. We aim to assess the quality of conduct, reporting, and interpretation of NITs that investigate the effects of surgical management in cancer patients.
Methods
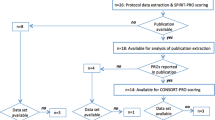
A cross-sectional analysis of papers identified through a comprehensive literature database search was performed. Forty papers employing a phase III noninferiority (NI) randomized trial design to study effects of surgical methodology or sequencing of surgery in patients with solid cancers were included. Papers were assessed for type of analysis, justification of the noninferiority margin (NIM), consistency of type I error with confidence intervals (CIs), ability to achieve the predefined sample size, and interpretations regarding NI.
Results
Only half of the papers used both intention-to-treat and per protocol analyses; 62.5% provided no or poor justification for the NIM; 42.5% showed inconsistency of the type I error rate with CIs; 52.5% were deemed poor or fair quality, and 60.0% did not achieve the predefined sample size. One-fifth of the papers provided interpretation of the NI hypothesis that was not in concordance with the CONSORT guidelines.
Conclusions
The quality of conduct, reporting, and interpretation of surgical NITs is suboptimal, requiring further improvements through adherence to guidelines and rigorous assessment at the stages of the study approval, funding, and the peer-review process.
Similar content being viewed by others
References
Huttner FJ, Capdeville L, Pianka F, et al. Systematic review of the quantity and quality of randomized clinical trials in pancreatic surgery. Br J Surg. Jan 2019;106(1):23–31.
Huttner FJ, Doerr-Harim C, Probst P, Tenckhoff S, Knebel P, Diener MK. Study methods in evidence-based surgery: methodological impediments and suggested approaches for the creation and transfer of knowledge in surgery. Eur Surg Res. 2014;53(1–4):86–94.
Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE—a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. Nov 2017;120(5):639–50.
Murthy VL, Desai NR, Vora A, Bhatt DL. Increasing proportion of clinical trials using noninferiority end points. Clin Cardiol. Sep 2012;35(9):522–3.
Suda KJ, Hurley AM, McKibbin T, Motl Moroney SE. Publication of noninferiority clinical trials: changes over a 20-year interval. Pharmacotherapy. 2011;31(9):833–9.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594–604.
Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ, Group C. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295(10):1152–60.
FDA. Non-Inferiority Clinical Trials to Establish Effectiveness, Guidance for Industry. Food and Drug Administration, US Department of Health and Human Services. 2016.
Aberegg SK, Hersh AM, Samore MH. Empirical consequences of current recommendations for the design and interpretation of noninferiority trials. J Gen Intern Med. 2018;33(1):88–96.
Rehal S, Morris TP, Fielding K, Carpenter JR, Phillips PP. Non-inferiority trials: are they inferior? A systematic review of reporting in major medical journals. BMJ Open. Oct 7 2016;6(10):e012594.
Turgeon RD, Reid EK, Rainkie DC. Design and interpretation of noninferiority trials. J Gen Intern Med. 2018;33(8):1215.
Acuna SA, Dossa F, Baxter NN. Frequency of misinterpretation of inconclusive noninferiority trials: the case of the laparoscopic vs open resection for rectal cancer trials. JAMA Surg. 2019;154(1):90–2.
Aberegg S. Reporting noninferiority trials. JAMA. 2013;309(15):1584–5.
Paesmans M, Grigoriu B, Ocak S, et al. Systematic qualitative review of randomised trials conducted in nonsmall cell lung cancer with a noninferiority or equivalence design. Eur Respir J. 2015;45(2):511–24.
Fueglistaler P, Adamina M, Guller U. Non-inferiority trials in surgical oncology. Ann Surg Oncol. 2007;14(5):1532–9.
2017 Journal Impact Factor, Journal Citation Reports, ClarivateAnalytics, 2018.
Acuna SA, Chesney TR, Amarasekera ST, Baxter NN. Defining non-inferiority margins for quality of surgical resection for rectal cancer: a Delphi consensus study. Ann Surg Oncol. 2018;25(11):3171–8.
Wyrwich KW, Spertus JA, Kroenke K, et al. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J. 2004;147(4):615–22.
Bellamy N, Carette S, Ford PM, et al. Osteoarthritis antirheumatic drug trials. III. Setting the delta for clinical trials–results of a consensus development (Delphi) exercise. J Rheumatol. 1992;19(3):451–7.
McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–3.
Acuna SA, Chesney TR, Baxter NN. Incorporating patient preferences in noninferiority trials. JAMA. 2019;322(4):305–6.
Llewellyn-Thomas HA, Williams JI, Levy L, Naylor CD. Using a trade-off technique to assess patients’ treatment preferences for benign prostatic hyperplasia. Med Decis Making. 1996;16(3):262–82.
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
D’Agostino RB, Sr., Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med. 2003;22(2):169–86.
Shrier I, Verhagen E, Stovitz SD. The intention-to-treat analysis is not always the conservative approach. Am J Med. 2017;130(7):867–71.
Matsuyama Y. A comparison of the results of intent-to-treat, per-protocol, and g-estimation in the presence of non-random treatment changes in a time-to-event non-inferiority trial. Stat Med. 2010;29(20):2107–16.
Head SJ, Kaul S, Bogers AJ, Kappetein AP. Non-inferiority study design: lessons to be learned from cardiovascular trials. Eur Heart J. 2012;33(11):1318–24.
Macaya F, Ryan N, Salinas P, Pocock SJ. Challenges in the design and interpretation of noninferiority trials: insights from recent stent trials. J Am Coll Cardiol. 2017;70(7):894–903.
Schmid SL. Five years post-DORA: promoting best practices for research assessment. Mol Biol Cell. 2017;28(22):2941–4.
Zhang L, Rousseau R, Sivertsen G. Science deserves to be judged by its contents, not by its wrapping: revisiting Seglen’s work on journal impact and research evaluation. PLoS One. 2017;12(3):e0174205.
DORA. San Francisco Declaration on Research Assessment. December 16, 2012, Accessed June 15, 2019.
Fung EK, Lore JM, Jr. Randomized controlled trials for evaluating surgical questions. Arch Otolaryngol Head Neck Surg. 2002;128(6):631–4.
McLeod RS. Issues in surgical randomized controlled trials. World J Surg. 1999;23(12):1210–4.
Yu J, Chen W, Chen S, et al. Design, conduct, and analysis of surgical randomized controlled trials: a cross-sectional survey. Ann Surg. 2019;270(6):1065–9.
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63(8):e1–37.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–75.
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–10.
Boutron I, Ravaud P. Misrepresentation and distortion of research in biomedical literature. Proc Natl Acad Sci USA. 2018;115(11):2613–9.
Boutron I, Haneef R, Yavchitz A, et al. Three randomized controlled trials evaluating the impact of “spin” in health news stories reporting studies of pharmacologic treatments on patients’/caregivers’ interpretation of treatment benefit. BMC Med. 2019;17(1):105.
Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–55.
Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–63.
Acknowledgments
We thank Mr. Toni Panzarella, M.Sc. (Biostatistics Division, Dalla Lana School of Public Health, University of Toronto, Ontario, Canada) for valuable discussion regarding the design of this study.
Funding
This study was sponsored by the internal funds from the Department of Surgery, University of Toronto.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Authors do not have any commercial interests in the subject of study and the source of any financial or material support.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parsyan, A., Marini, W., Fazelzad, R. et al. Current Issues in Conduct and Reporting of Noninferiority Randomized Controlled Trials in Surgical Management of Cancer Patients. Ann Surg Oncol 28, 39–47 (2021). https://doi.org/10.1245/s10434-020-08575-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08575-7