Abstract
Background
Many inflammatory markers can be used for the prognostication of pancreatic cancer, but which combination of inflammatory factors may be the best remains unclear. This study focused on the potential feasibility of the newly discovered C-reactive protein (CRP)/lymphocyte ratio (CLR) as a prognostic biomarker for patients with pancreatic cancer.
Methods
The study enrolled 997 patients with pancreatic cancer. Six combinations of inflammatory markers, namely, the neutrophil/lymphocyte ratio (NLR), the platelet/lymphocyte ratio (PLR), the CRP/albumin ratio (CAR), the neutrophil/albumin ratio (NAR), the platelet/albumin ratio (PAR), and CLR, were examined to determine which combination offers the highest accuracy for predicting poor survival by receiver operating characteristic curve analysis. The prognostic value of the CLR was analyzed by uni- and multivariate analyses.
Results
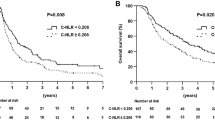
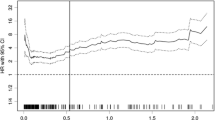
The newly developed CLR was more accurate than the NLR, PLR, CAR, NAR, and PAR in predicting survival. The optimal cutoff value for the CLR was calculated to be 1.8 for survival. A CLR higher than 1.8 was associated with poor survival in both the univariate (hazard ratio [HR] 2.00; P < 0.001) and multivariate (HR 1.73; P < 0.001) analyses. In addition, a CLR higher than 1.8 was an independent risk factor for patients with stage 2 (HR 1.85; P = 0.001), stage 3 (HR 1.83; P = 0.001), or stage 4 (HR 1.70; P < 0.001) disease.
Conclusions
Pretreatment CLR can be considered a feasible biomarker for the prognostic prediction of pancreatic cancer. An elevated CLR was an independent risk factor for poor survival, with a cutoff value of 1.8.



Similar content being viewed by others
References
Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11–26.
Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–20.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Sidaway P. Pancreatic cancer: TCGA data reveal a highly heterogeneous disease. Nat Rev Clin Oncol. 2017;14:648.
Fahrmann JF, Bantis LE, Capello M, et al. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J Natl Cancer Inst. 2018;111:372–9.
Luo G, Liu C, Guo M, et al. Potential biomarkers in Lewis-negative patients with pancreatic cancer. Ann Surg. 2017;265:800–5.
Nahm CB, Turchini J, Jamieson N, et al. Biomarker panel predicts survival after resection in pancreatic ductal adenocarcinoma: a multi-institutional cohort study. Eur J Surg Oncol. 2018;45:218–24.
Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio: a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2016;24:561–8.
Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–6.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41.
Taniguchi K, Karin M. NF-κB, inflammation, immunity, and cancer: coming of age. Nat Rev Immunol. 2018;18:309–24.
Imaoka H, Mizuno N, Hara K, et al. Evaluation of modified glasgow prognostic score for pancreatic cancer: a retrospective cohort study. Pancreas. 2015;45:211–7.
Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601–20.
Tomioka N, Azuma M, Ikarashi M, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer. 2018;25:34–42.
Kuznetsov HS, Marsh T, Markens BA, et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov. 2012;2:1150–65.
Gunter MJ, Tao W, Mary C, et al. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst. 2016;107:djv169.
Hooman D, Harman Maxim B, Gus M, et al. The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. 2014;113:887–93.
Templeton AJ, Knox JJ, Lin X, et al. Change in neutrophil-to-lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol. 2016;70:358–64.
Cummings M, Merone L, Keeble C, et al. Preoperative neutrophil: lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113:311–20.
Guo S, He X, Chen Q, et al. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC Cancer. 2017;17:171.
Kawai M, Hirono S, Okada KI, et al. Low lymphocyte-monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery. 2019;165:1151–60.
Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol. 2018;25:845–7.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89.
Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–96.
Lee BM, Chung SY, Chang JS, et al. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12:342–52.
Tingle SJ, Severs GR, Goodfellow M, et al. NARCA: a novel prognostic scoring system using neutrophil-albumin ratio and Ca19-9 to predict overall survival in palliative pancreatic cancer. J Surg Oncol. 2018;118:680–6.
Gershov D, Kim S, Brot N, et al. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exper Med. 2000;192:1353–64.
Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflamm Res. 2019;68:815–23.
Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology. 2015;15:694–700.
Miyamoto R, Oda T, Hashimoto S, et al. Platelet × CRP multiplier value as an indicator of poor prognosis in patients with resectable pancreatic cancer. Pancreas. 2017;46:35–41.
Shirin K, Subhasis C, Chakraborty NG. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology. 2015;144:186–96.
Clark EJ, Connor S, Taylor MA, et al. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB. 2007;9:456–60.
Rho SY, Hwang HK, Chong JU, et al. Association of preoperative total lymphocyte count with prognosis in resected left-sided pancreatic cancer. ANZ J Surg. 2019;89:503–8.
Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2019. https://doi.org/10.1097/SLA.0000000000003239.
Nozoe T, Kono M, Kuma S, et al. New scoring system to create a prognostic criteria in colorectal carcinoma based on serum elevation of C-reactive protein and decrease in lymphocyte in peripheral blood. JMI J Med Invest. 2019;66:264–8.
Templeton AJ, Mcnamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81625016, 81871940, 81902417), the Shanghai Natural Science Foundation (Grant No. 17ZR1406300), the Shanghai Cancer Center Foundation for Distinguished Young Scholars (Grant No. YJJQ201803), and the Fudan University Personalized Project for “Double Top” Original Research (Grant No. XM03190633).
Author information
Authors and Affiliations
Contributions
CL and XY designed the study, and ZF, YG and GL performed the statistical analysis. All authors collected the clinical data and drafted the manuscript. XY and CL critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Kaplan–Meier plot of overall survival (OS) between patients with a C-reactive protein/lymphocyte ratio (CLR) higher than 1.8 and patients with a CLR of 1.8 or lower in the (a) surgical and (b) nonsurgical groups. (TIFF 665 kb)
Rights and permissions
About this article
Cite this article
Fan, Z., Luo, G., Gong, Y. et al. Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Ann Surg Oncol 27, 4017–4025 (2020). https://doi.org/10.1245/s10434-020-08301-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08301-3