Abstract
Introduction
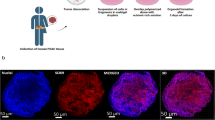
We hypothesized that engineering a combined lymph node/melanoma organoid from the same patient would allow tumor, stroma, and immune system to remain viable for personalized immunotherapy screening.
Methods
Surgically obtained matched melanoma and lymph node biospecimens from the same patient were transferred to the laboratory and washed with saline, antibiotic, and red blood cell lysis buffer. Biospecimens were dissociated, incorporated into an extracellular matrix (ECM)-based hydrogel system, and biofabricated into three dimensional (3D) mixed melanoma/node organoids. Cells were not sorted, so as to preserve tumor heterogeneity, including stroma and immune cell components, resulting in immune-enhanced patient tumor organoids (iPTOs). Organoid sets were screened in parallel with nivolumab, pembrolizumab, ipilimumab, and dabrafenib/trametinib for 72 h. LIVE/DEAD staining and quantitative metabolism assays recorded relative drug efficacy. Histology and immunohistochemistry were used to compare tumor melanoma cells with organoid melanoma cells. Lastly, node-enhanced iPTOs were employed to activate patient-matched peripheral blood T cells for killing of tumor cells in naïve PTOs.
Results
Ten biospecimen sets obtained from eight stage III and IV melanoma patients were reconstructed as symbiotic immune/tumor organoids between September 2017 and June 2018. Successful establishment of viable organoid sets was 90% (9/10), although organoid yield varied with biospecimen size. Average time from organoid development to initiation of immunotherapy testing was 7 days. In three patients for whom a node was not available, it was substituted with peripheral blood mononuclear cells. iPTO response to immunotherapy was similar to specimen clinical response in 85% (6/7) patients. In an additional pilot study, peripheral T cells were circulated through iPTOs, and subsequently transferred to naïve PTOs from the same patient, resulting in tumor killing, suggesting a possible role of iPTOs in generating adaptive immunity.
Conclusion
Development of 3D mixed immune-enhanced tumor/node organoids is a feasible platform, allowing individual patient immune system and tumor cells to remain viable for studying of personalized immunotherapy response.




Similar content being viewed by others
References
Yan HHN, Siu HC, Law S, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 2018; 23: 882–897 e811.
Song L, Dong G, Guo L, Graves DT. The function of dendritic cells in modulating the host response. Mol Oral Microbiol 2018; 33: 13–21.
Neal JT, Li X, Zhu J, et al. Organoid modeling of the tumor immune microenvironment. Cell 2018; 175: 1972–1988 e1916.
Skardal A, Zhang J, McCoard L, et al. Dynamically crosslinked gold nanoparticle - hyaluronan hydrogels. Adv Mater 2010; 22: 4736–4740.
Murphy SV, Skardal A, Song L, et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full-thickness wound healing. Stem Cells Transl Med 2017; 6: 2020–2032.
Clark CC, Aleman J, Mutkus L, Skardal A. A mechanically robust thixotropic collagen and hyaluronic acid bioink supplemented with gelatin nanoparticles. Bioprinting 2019; 16.
Mazzocchi A, Devarasetty M, Huntwork R, et al. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018; 11: 015003.
Skardal A, Devarasetty M, Soker S, Hall AR. In situ patterned micro 3D liver constructs for parallel toxicology testing in a fluidic device. Biofabrication 2015; 7: 031001.
Skardal A, Murphy SV, Devarasetty M, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 2017; 7: 8837.
Zhang YS, Aleman J, Shin SR, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A 2017; 114: E2293–E2302.
Skardal A, Devarasetty M, Kang HW, et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater 2015; 25: 24–34.
Aleman J, Skardal A. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells. Biotechnol Bioeng 2018.
Aleman J, George SK, Herberg S, et al. Deconstructed microfluidic bone marrow on-a-chip to study normal and malignant hemopoietic cell-niche interactions. Small 2019; epub ahead of print.
Mazzocchi AR, Rajan SAP, Votanopoulos KI, et al. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep 2018; 8: 2886.
Mazzocchi A, Devarasetty M, Herberg S, et al. Pleural effusion aspirate for use in 3d lung cancer modeling and chemotherapy screening. ACS Biomater Sci Eng 2019; 5: 1937–1943.
Votanopoulos KI, Mazzocchi A, Sivakumar H, et al. Appendiceal cancer patient-specific tumor organoid model for predicting chemotherapy efficacy prior to initiation of treatment: a feasibility study. Ann Surg Oncol 2019; 26: 139–147.
Forsythe S, Mehta N, Devarasetty M, et al. Development of a colorectal cancer 3D micro-tumor construct platform from cell lines and patient tumor biospecimens for standard-of-care and experimental drug screening. Ann Biomed Eng 2019.
Skardal A, Devarasetty M, Forsythe S, et al. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng 2016; 113: 2020–2032.
Skardal A, Smith L, Bharadwaj S, et al. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials 2012; 33: 4565–4575.
Mazzocchi AR, Soker S, Skardal A. Biofabrication technologies for developing in vitro tumor models. In Soker S, Skardal A (eds): Tumor Organoids. Berlin, Germany: Springer Nature 2017; 51–70.
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and Phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33: 1889–1894.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373: 23–34.
Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018; 174: 1586–1598 e1512.
Acknowledgements
We wish to thank Libby McWilliams (Procurement Manager), Kathleen Cummings (Protocol and Data Manager), and the Wake Forest Advanced Tumor Bank Shared Resource. A.S. acknowledges funding through the Wake Forest Clinical and Translational Science Institute Open Pilot Program, supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through grant award no. UL1TR001420. A.S. and K.I.V. acknowledge funding through the Comprehensive Cancer Center at Wake Forest Baptist Medical Center’s Clinical Research Associate Director Pilot Funds, and services from the Tumor Tissue and Pathology Shared Resource supported by the Comprehensive Cancer Center at Wake Forest Baptist Medical Center’s NCI Cancer Center Support Grant P30CA012197.
Funding
The work was supported by Wake Forest Comprehensive Cancer Center Pilot Fund.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Votanopoulos, K.I., Forsythe, S., Sivakumar, H. et al. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann Surg Oncol 27, 1956–1967 (2020). https://doi.org/10.1245/s10434-019-08143-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08143-8