Abstract
Background
This study aimed to evaluate whether rapid fluorescent tissue examination immediately after colorectal cancer liver metastasis (CLM) ablation correlates with standard pathologic and immunohistochemical (IHC) assessments.
Methods
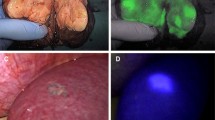
This prospective, National Institutes of Health-supported study enrolled 34 consecutive patients with 53 CLMs ablated between January 2011 and December 2014. Immediately after ablation, core needle sampling of the ablation zone was performed. Tissue samples were evaluated with fluorescent viability (MitoTracker Red) and nuclear (Hoechst) stains. Confocal microscope imaging was performed within 30 min after ablation. The same samples were subsequently fixed and stained with hematoxylin and eosin (H&E). Identified tumor cells underwent IHC staining for proliferation (Ki67) and viability (OxPhos). The study pathologist, blinded to the H&E and IHC assessment, evaluated the fluorescent images separately to detect viable tumor cells. Sensitivity, specificity, and overall concordance of the fluorescent versus H&E and IHC assessments were calculated.
Results
A total of 63 tissue samples were collected and processed. The overall concordance rate between the immediate fluorescent and the subsequent H&E and IHC assessments was 94% (59/63). The fluorescent assessment sensitivity and specificity for the identification of tumor cells were respectively 100% (18/18) and 91% (41/45).
Conclusions
The study showed a high concordance rate between the immediate fluorescent assessment and the standard H&E and IHC assessment of the ablation zone. Given the documented prognostic value of ablation zone tissue characteristics for outcomes after ablation of CLM, the fluorescent assessment offers a potential intra-procedural biomarker of complete tumor ablation.


Similar content being viewed by others
References
Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. http://www.ncbi.nlm.nih.gov/pubmed/20496531.
Arnaud JP, Dumont P, Adloff M, Leguillou A, Py JM. Natural history of colorectal carcinoma with untreated liver metastases. Surg Gastroenterol. 1984;3:37–42. http://www.ncbi.nlm.nih.gov/pubmed/6522907.
Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15(Suppl 4):iv103–6. https://doi.org/10.1093/annonc/mdh912.
Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorectal Cancer. 2011;10:258–65. https://doi.org/10.1016/j.clcc.2011.06.008.
Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes: a 10-year experience at a single center. Radiology. 2016;278:601–11. https://doi.org/10.1148/radiol.2015142489.
Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radiofrequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–66. https://doi.org/10.1148/radiol.2211001624.
Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer. 2004;4:92–100. https://doi.org/10.3816/ccc.2004.n.012.
White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg. 2007;11:256–63. https://doi.org/10.1007/s11605-007-0100-8.
Cornelis F, Sotirchos V, Violari E, et al. 18F-FDG PET/CT is an immediate imaging biomarker of treatment success after liver metastasis ablation. J Nucl Med. 2016;57:1052–7. https://doi.org/10.2967/jnumed.115.171926.
Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36:166–75. https://doi.org/10.1007/s00270-012-0377-1.
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68. https://doi.org/10.1148/radiol.12111851.
Sotirchos VS, Petrovic LM, Gönen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–59. https://doi.org/10.1148/radiol.2016151005.
Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol. 2012;19:4262–9. https://doi.org/10.1245/s10434-012-2461-9.
Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol. 2013;20(3 Suppl):S676–83. https://doi.org/10.1245/s10434-013-3140-1.
Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology. 2008;249:364–74. https://doi.org/10.1148/radiol.2491071752.
Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol. 2011;18:3702–10. https://doi.org/10.1245/s10434-011-1762-8.
Snoeren N, Jansen MC, Rijken AM, et al. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg. 2009;26:56–62. https://doi.org/10.1159/000194946.
Tanis E, Spliethoff JW, Evers DJ, et al. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur J Surg Oncol. 2015;42:251–9. https://doi.org/10.1016/j.ejso.2015.12.005.
Ryan ER, Sofocleous CT, Schöder H, et al. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology. 2013;268:288–95. https://doi.org/10.1148/radiol.13121462.
Sofocleous CT, Sideras P, Petre EN. How we do it: a practical approach to hepatic metastases ablation techniques. Tech Vasc Interv Radiol. 2013;16:219–29. https://doi.org/10.1053/j.tvir.2013.08.005.
Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria: a 10-year update. Radiology. 2014;273:241–60. https://doi.org/10.1148/radiol.14132958.
Boffa DJ, Waka J, Thomas D, et al. Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplantation. 2005;79:842–5. https://doi.org/10.1097/01.tp.0000155175.24802.73.
Fujisawa S, Romin Y, Barlas A, et al. Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases. Cytotechnology. 2014;66:259–73. https://doi.org/10.1007/s10616-013-9565-3.
Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–63. https://doi.org/10.1002/1097-0142(20000601)88:11%3c2452::aid-cncr5%3e3.0.co;2-3.
Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases: analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014;50:912–9. https://doi.org/10.1016/j.ejca.2013.12.008.
Mbah NA, Scoggins C, McMasters K, Martin R. Impact of hepatectomy margin on survival following resection of colorectal metastasis: the role of adjuvant therapy and its effects. Eur J Surg Oncol. 2013;39:1394–9. https://doi.org/10.1016/j.ejso.2013.09.009.
Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–75. https://doi.org/10.1016/j.jvir.2017.08.021.
Shady W, Petre EN, Vakiani E, et al. Kras mutation is a marker of worse oncologic outcomes after percutaneous radiofrequency ablation of colorectal liver metastases. Oncotarget. 2017;8:66117–27. https://doi.org/10.18632/oncotarget.19806.
Ziv E, Bergen M, Yarmohammadi H, et al. PI3K pathway mutations are associated with longer time to local progression after radioembolization of colorectal liver metastases. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.15278.
Calandri M, Yamashita S, Gazzera C, et al. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018. https://doi.org/10.1007/s00330-017-5273-2.
Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104:760–8. https://doi.org/10.1002/bjs.10490.
Acknowledgment
This study was supported by the National Cancer Institute (Grant R21 CA131763-01A1). We gratefully acknowledge Alessandra Garcia, BA (Research Study Assistant at Memorial Sloan Kettering Cancer Center) for her contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
This study was funded in part by NIH/NCI R21 CA131763-01A1 grant and it was presented at the RSNA annual meeting in Chicago November 28th–December 3rd, 2014. Memorial Sloan Kettering Cancer Center is supported by the grant P30 CA008748 from the National Cancer Institute (NCI). Authors Vlasios S. Sotirchos, Sho Fujisawa, Efsevia Vakiani and Katia O. Manova-Todorova indicated no conflict of interest (COI) disclosures to report. Dr. Stephen B. Solomon has reported the following industry relations and potential COI: Consulting: BTG, Johnson & Johnson, Adgero, Aperture Medical, XACT Robotics, Innoblative. Research Support: GE Healthcare, AngioDynamics, Johnson & Johnson, Elesta. Shareholder: Aspire Bariatrics, Aperture Medical, Johnson & Johnson, Immunomedics. Dr. Constantinos T. Sofocleous has reported the following industry relations and potential COI: Consulting: Neuwave/Ethicon Johnson & Johnson, Terumo Medical, GE Healthcare. Research Support: Neuwave/Ethicon Johnson & Johnson; Angiodynamics, HS Medical, BTG.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sotirchos, V.S., Fujisawa, S., Vakiani, E. et al. Fluorescent Tissue Assessment of Colorectal Cancer Liver Metastases Ablation Zone: A Potential Real-Time Biomarker of Complete Tumor Ablation. Ann Surg Oncol 26, 1833–1840 (2019). https://doi.org/10.1245/s10434-018-07133-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-018-07133-6