Abstract
Hot-melt extrusion (HME) is a globally recognized, robust, effective technology that enhances the bioavailability of poorly soluble active pharmaceutical ingredients and offers an efficient continuous manufacturing process. The twin-screw extruder (TSE) offers an extremely resourceful customizable mixer that is used for continuous compounding and granulation by using different combinations of conveying elements, kneading elements (forward and reverse configuration), and distributive mixing elements. TSE is thus efficiently utilized for dry, wet, or melt granulation not only to manufacture dosage forms such as tablets, capsules, or granule-filled sachets, but also for designing novel formulations such as dry powder inhalers, drying units for granules, nanoextrusion, 3D printing, complexation, and amorphous solid dispersions. Over the past decades, combined academic and pharmaceutical industry collaborations have driven novel innovations for HME technology, which has resulted in a substantial increase in published articles and patents. This article summarizes the challenges and models for executing HME scale-up. Additionally, it covers the benefits of continuous manufacturing, process analytical technology (PAT) considerations, and regulatory requirements. In summary, this well-designed review builds upon our earlier publication, probing deeper into the potential of twin-screw extruders (TSE) for various new applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
The pharmaceutical industry is undoubtedly intrigued by hot-melt extrusion (HME) technology, as evidenced by the substantial increase in patents and publications. HME is rapidly becoming the preferred choice in the pharmaceutical industry due to its solvent-free and eco-friendly approach to continuous manufacturing [1, 2]. HME has proven highly effective in improving the solubility and dissolution of poorly water-soluble drugs by creating amorphous solid dispersions. Additionally, it is an essential tool for producing innovative pharmaceutical products [3, 4]. During the process, the active pharmaceutical ingredient is mixed at a molecular level with thermoplastic binders and/or polymers while passing through high-temperature counter-rotating or corotating screw elements. This meticulous molecular mixing process transforms the active pharmaceutical ingredient into an amorphous form, enhancing the dissolution rate [5, 6]. HME offers numerous advantages over traditional methods, as previously highlighted in part of this review titled “Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation in AAPS PharmSciTech, 2016” [6].
In our earlier published review, the authors explored the intricacies of HME technology, discussing the various types of process technology and equipment, types of screw extruders, principles of operation, and raw materials used. Our insights with multiple case studies back it up and explore the diverse range of pharmaceutical applications of HME. Furthermore, the article highlighted the advantages of HME as a continuous pharmaceutical manufacturing process while also addressing the challenges of scaling up and optimizing the process using QBD approaches.
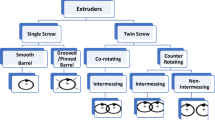
Extruders are designed as single-screw, twin-screw, or multi-screw extruders, and any extruder must meet regulatory standards for pharmaceutical dosage manufacturing. The twin-screw extruders (TSE) offer superior performance in the pharmaceutical manufacturing sector, as discussed in our previous review. This well-constructed review builds upon our earlier publication, delving deeper into the potential of TSE for various new applications. These applications include the creation of dry powder inhalers, TSE as effective drying equipment, nanoextrusion, cocrystals, complexation, and solid dispersion. Additionally, this review covers various types of twin-screw granulation and equipment modifications and provides relevant case studies. The section on HME as a continuous manufacturing process has been significantly expanded to include scale-up considerations, process analytical technology (PAT) considerations, and valuable insights into marketed products.
Tools Used to Prepare the Review
Various search engines, such as Google Scholar, PubMed, and ResearchGate, primarily focused on Google Scholar due to its widespread usage, were employed to explore original research and review articles. The broad search strategy encompassed various relevant articles, ensuring comprehensive coverage. Specific criteria were established to select papers, focusing on peer-reviewed articles with substantial citations from peer-reviewed journals. The search utilized keywords such as “Twin Screw Extrusion,” “Twin screw granulation,” “Hot-Melt Extrusion,” “Twin screw extrusion pharmaceutical applications,” “Hot-Melt extrusion + Scale-up,” “HME coupled fused deposition modeling 3D printing,” “HME + continuous manufacturing,” “HME + PAT tools,” etc. Additionally, a supplementary search was conducted in review papers and Google patents to identify formulations manufactured using Hot-Melt Extrusion that were marketed. Manual titles and abstracts were screened to identify the most relevant information and essential case studies.
Pharmaceutical Applications of Twin-Screw Extruders
Significant progress has been observed in developing novel applications using TSE technology, which is also reflected by the increased number of recent publications and patents in this area. Several novel applications of TSE are discussed in the following section.
TSE as a Mixer for Dry Powders for Inhalation
Dry powder inhalers have gained popularity as a delivery method due to their numerous advantages over the years. In this system, a larger quantity of coarser excipient particles is mixed with a smaller portion of a potent drug of relatively finer particles [7]. Moreover, the drug’s performance and lung delivery rely on adhesion forces with the excipient. Therefore, achieving a homogenous blend uniformity is critical in developing dry powder inhalation dosage forms [7]. Different techniques have been developed to achieve a uniform distribution of the drug in the final dosage form. However, these methods may experience batch-to-batch variations due to inadequate mixing. Using HME as a mixer for dry powders can overcome this challenge and enable continuous manufacturing by dispensing the blend into final containers with proper setup [8]. Twin-screw extruders offer the opportunity to continuously manufacture dry powders for the inhalation [8]. Recently, Ren et al. used a twin-screw co-rotating extruder as a continuous mixer for blending powders for inhalation [9]. The authors aimed to reduce batch-to-batch variations when blending in tumble or high-shear batch mixers. The screw profile can be seen in Fig. 1a. The extruder was fed using two volumetric twin-screw feeders, one containing the API and the other containing the excipients. Then, both streams were blended in the extruder. The combing mixing elements blended the powder streams with high efficiency due to the axial slits that can be observed in Fig. 1. The extruder-blended powders had similar aerosol performance to those blended using high-shear batch mixing, and in the extruder, the mixing was done in less than 2 min [9]. As mentioned, the authors used an intermeshing twin-screw co-rotating extruder, a “self-wiping” setting, that has traditionally demonstrated reduced losses during production [10, 11]. In their following research article, the authors evaluated the effect of employing different mixing elements and processing parameters on the uniformity and aerosol performance of the powders for inhalation. Among the findings, the authors demonstrated that the use of 30° kneading elements was able to obtain better homogeneity than using the combing mixing elements employed in their previous publication [9]. Regarding aerosol performance, there were no differences between combing mixing and the 30° kneading elements for all feed rates/screw speeds tested until reaching too high specific energies (> 10 g/min and 500 rpm). The behavior of powder mixing on the twin-screw mixer is interesting, especially when examining differences between segregating and non-segregating powders. According to Pilcer et al., non-segregating powders may require a longer mixing time to achieve the desired results. On the other hand, segregating powders tends to stick together in clumps, and a specific mixing time is necessary to break them apart for optimal results [12]. This information highlights the importance of understanding the properties of different types of powders and tailoring the mixing process accordingly.
This approach using TSE for mixing the different components can effectively avoid a unit operation to make pre-blends before the extrusion process by feeding formulation components separately into the extruder (Fig. 2).
Twin Screw Extruders as Drying Equipment
Drying the materials is critical in commercial manufacturing, and it requires thermal energy. In the manufacturing of solid dosage forms, drying the API is a crucial step. If drying is not controlled, inappropriate morphology of particles or an undetermined polymorph state can occur. During the drying process, several phenomena occur, such as agglomeration, attrition, (re)-crystallization, and redissolution. Agglomeration produces larger particles, which leads to slower dissolution, while attrition produces smaller particles, resulting in poor flowability [13]. The drying process reduces particle size via shearing or induced stresses caused by particle collisions. It also influences the particle size distribution of the final product. Additionally, the residual moisture content in the final product causes instability of the active pharmaceutical products. Several techniques have been employed to dry the material, such as tray drying, fluid-bed systems, spray drying, and paddle drying. However, drying smaller particle-size material with poor flowability using the existing technologies is very challenging. Therefore, hot-melt extrusion can address these constraints along with continuous manufacturing. The twin-screw extruder is a highly effective drying technology that outperforms other methods like fluidized bed dryers. It ensures precise temperature control and can dry materials below their glass transition temperature, leading to minimal/no particle size and quality changes. Additionally, the extruder’s residence time has little impact on reducing particle size [14].
In the pursuit of the transition from batch manufacturing toward continuous manufacturing, wet granulation is particularly challenging because it requires several unit operations [15]. A twin-screw extruder can easily combine the blending using a multi-feeder approach, as shown in Fig. 3, and granulation steps into one unit operation [16]. However, the drying unit operation is more challenging for an extruder [15]. In recent years, several research groups have envisioned using a twin-screw extruder to simultaneously perform wet granulation and drying in one single and continuous unit operation.
Meena et al. (2017) optimized extruder parameters and were able to produce acetaminophen granules with moisture of 0.1% w/w. These granules did not require milling or sieving before tableting. Schmidt et al. (2018) studied in depth the drying capabilities of twin screw granulation, operating at higher temperatures and with higher amounts of water than the study reported by Meena et al. (2017) [16]. The authors compared the granules produced by a corotating Pharma 11 mm extruder with a fluidized bed dryer. The authors studied variables such as mass flow rate, L/S ratio, screw speed, and temperature in different extruder zones. One of the most critical findings is that screw speed has a significant impact on controlling heat transfer by modifying the residence time of the material in the extruder. Additionally, the study found differences in disintegration when comparing the granules from in-barrel-drying and fluidized-bed drying [16].
In a subsequent research article, Schmidt A et al. (2018) aimed to perform wet granulation and drying using an API suspension instead of solid feeding. They aimed to reduce the unit operations needed to transition from primary to secondary manufacturing. The authors demonstrated that it is feasible to combine API crystallization and drying (primary manufacturing) with wet granulation and drying (secondary manufacturing), performing several operation units in one step [13]. Kreimer et al. (2018) employed a twin-screw extruder to dry a crystallized product during primary manufacturing with a similar purpose because improper drying can lead to changes in particle size distribution and morphologic changes that can negatively impact further manufacturing of an API [17].
It is known that process validation must be pursued in each production scale, and usually, the optimized parameters from a laboratory scale are not transferrable to larger scales. Haser et al. (2023) claimed they were the first to achieve simultaneously wet granulation and drying in a single unit operation using a pilot scale twin-screw extruder (ZSE HP-18, American Leistritz Extruder Corp). In their barrel configuration, the authors tested different screw designs and the addition/position of vacuums in the vents, significantly impacting moisture removal. Additionally, the authors were able to monitor the process in-line using the NIR spectroscopy [18].
Nanoextrusion
One of the most common uses of twin-screw extruders is for devolatilization purposes. Through the venting zones, it is possible to remove water, solvents, and other volatile compounds during processing [11]. The concept of nano extrusion implies using an extruder to remove the solvent from nanosuspensions while dispersing the nanoparticles in a polymeric matrix, forming a nanocomposite [19]. This technique addresses nanosuspension stability issues due to particle aggregation from Ostwald ripening, which can occur during processing, storage, and handling of the suspension [20]. Additionally, this strategy of dispersing a nanosuspension in an extrudate increases the dissolution rate due to improved wettability while maintaining the benefits of nanoparticles related to reduced particle size and increased surface area [21].
It is important to mention that during nanoextrusion, the processing temperature must be lower than the melting point of the chosen drug to avoid the generation of an ASD [19]. Most of the published research articles on nanoextrusion employed Soluplus® for stabilizing the nanoparticles in solid-state, likely due to its lower Tg compared to other water-soluble polymers commonly used during HME [19,20,21,22].
Khinast et al. (2013) published the first work using the term nanoextrusion. In that rapid communication, the authors aimed to homogeneously distribute nanocrystals (TiO2) in a water-soluble polymeric matrix (Soluplus®) using a ZSK 18 twin screw extruder (Coperion GmbH, Stuttgart, Germany) with a ten-zone barrel. Soluplus® was fed in zone 1, while zone 4 was left as an atmospheric degassing section to remove the polymer’s moisture. Then, the nanosuspension was added at zone 5 to the molten material, and the solvent was removed in zone 8 (devolatilization), as shown in Fig. 4a. According to the authors, it was possible to add as much as 30% (w/w) of water to the molten material where complete solvent removal was still possible [20].
Li et al. (2010) used a Nano-16 co-rotating twin-screw extruder (Leistritz Extrusionstechnik GmbH, Nürnberg, Germany) at a screw speed of 50 rpm for the preparation of nanocomposites and amorphous solid dispersions (ASD) of griseofulvin. Interestingly, griseofulvin is miscible in Soluplus®, leading to the generation of ASD. To prepare a nanocomposite, the authors considered the differences of drug–polymer miscibility and employed hydroxypropyl cellulose (HPC) to avoid the generation of an ASD and disperse the nanosuspension in the polymeric matrix. Figure 4b shows that zone 1 was equipped with conveying elements at 60 °C. The nanosupension was added to zone 2, at 90 °C, right before the kneading elements (60°offset angle). Zone 3 was equipped with conveying elements, similar to zone 1, but in this case, the temperature was set at 150 °C. The die was kept at 160 °C. In this setting, the removal of water was in the mixing zones, where the vent ports are kept open [23].
In another example of an extruder setting, Ye et al. (2016) employed an 11-mm co-rotating twin-screw extruder (Thermo Fisher Scientific, Waltham, MA, USA) to prepare a nanocomposite of efavirenz [21]. In this eight-zone extruder, the screw speed was set at 50 rpm, the barrel was kept in the range of 100–140 °C, and the nanosuspension was added in zone 5. The configuration of the screws can be found in Fig. 4c. The first zone was fed with the polymer (Soluplus®) and was equipped with conveying elements (long helix feed screw, 2.0 L/D), providing enough free volume to incorporate the material. Then, in zone 2, a different type of conveying element (feed screw, 1.0 L/D) was employed to compact and push forward the polymer. Zone 3 comprised mixing elements with 30°, 60°, and 90° offset angles, and zones 4 and 5 comprised conveying elements (feed screw, 1.0 L/D). The efavirenz nanosuspension was incorporated in zone 5, which was kept at 110 °C. This configuration forces the water to move between zones 4 and 5 until evaporation. Zone 6 was equipped with mixing elements with offset angles of 30° and 60° to enhance the distribution of the nanosuspension in the polymeric sample. However, the authors did not employ mixing elements with an offset angle of 90° to avoid efavirenz’s amorphization. Zones 7 and 8 were equipped with conveying elements (feed screw, 1.0 L/D). The generated nanocomposite was stable for at least six months, along with stable particle size, PDI, and zeta potential of the nanosuspension [21].
Twin Screw Granulation
The use of TSE technology in granulation has gained interest from both pharmaceutical academics and industry experts. As a result, twin-screw granulation (TSG) has been recognized as a viable alternative approach and is now being implemented in commercial continuous manufacturing processes. The TSG technique is a modern granulation method that provides various advantages, including reduced processing time and cost, compatibility with continuous manufacturing processes, and effective handling of high drug load formulations with poor flow properties. It requires less binder to produce equivalent granules than batch systems, minimizes lot-to-lot variation of the granulated product, and eliminates the need for a seasoned operator.
The process of twin-screw granulation can be categorized into three types based on temperature and binder utilization: twin-screw dry granulation (TSDG), twin-screw wet granulation (TSWG), and twin-screw melt granulation (TSMG) [24].
All three types of granulations result in dense granules that have good flowability, compressibility, and homogeneity. However, the properties of the final granules may differ depending on various factors such as that related to equipment, formulation, and process considerations [25]. All three types of twin-screw granulation could be widely used for various pharmaceutical applications. Table I shows a comparison of different types of TSG with conventional HME for its pharmaceutical applications.
Factors Influencing Granular Properties
Equipment Considerations
TSG is constructed with conveying elements, mixing (wide-toothed, narrow-toothed, comb mixer, screw mixing) elements, and kneading elements. The selection of these elements is crucial based on the type of TSG (Fig. 5). The kneading block has garnered the highest interest of all screw elements. Its compressive forces primarily stem from two factors: (i) the reduced accessible volume and (ii) the limited ability to transport powders, known as drag flow. Instead, the mechanism relies on the pressure generated along the screw element to propel granules forward, a phenomenon called pressure-driven flow. Typically, these kneading blocks are available with four offset angle choices: 30°, 45°, 60°, and 90°. The smaller angles provide increased capacity for drag flow (i.e., pushing behavior), resembling a conveying element. The screw configuration (number of mixing or kneading zones) is also critical based on the type of TSG. The size and shape of elements, flight pitch, flight depth, and the gap between the screw-screw and screw-barrel play a vital role in end product (granule) quality. A higher offset angle resulted in larger, denser granules with higher channel fill rates [25]. J. Vercruysse et al. studied the impact of screw configuration on the particle size distribution (PSD) of granules produced by twin-screw wet granulation, where the researcher found out that combining kneading elements with screw mixing elements could result in good quality of granules with narrow PSD [26].
Formulation Considerations
Selecting the appropriate API, binder/polymer, and other excipients is crucial for a specific type of TSG. Excipients like fillers or diluents, disintegrants, and other functional ingredients should be carefully chosen based on their compatibility with the drug and binder and their impact on the granule properties (Fig. 5).
The binder system, which is typically either lipid-based or polymer-based, dictates the attributes of the final granules. The selection of the binder system depends on factors such as drug-binder compatibility, melting point, viscosity, and desired release characteristics [24]. Binders with low Tg and low viscosity are suitable for dry and wet granulations, respectively. For TSMG, it is desired to have a low melting point, small particle size, and low viscosity binder to achieve good granules with less percentage of fines. This is because of the distribution mechanism of the binder within the extruder. Commonly used binders include hydroxypropyl methylcellulose (HPMC) and hydroxypropyl cellulose (HPC), as well as synthetic polymers such as polyvinylpyrrolidone (PVP) [27].
Diluent should be selected based on the formulation’s polymer/plasticizer/lipid concentration. If the concentration of melting material is high, diluent with high adsorption capacity is suitable to get the granules with good compressibility. The most used diluents are MCC, Lactose, Mannitol, and Dicalcium phosphate [28]. Disintegrants may be used for immediate-release formulations. A disintegrating agent may not be required if the diluent (MCC) has the disintegration property. For the TSWG L/S ratio, the viscosity of the liquid binder and the molecular weight of the binder are critical formulation variables to be considered.
Process Considerations
TSG is a complex process that involves various variables that can be adjusted to optimize the formulation and manufacturing of granules. These process variables may impact the final granule properties (Fig. 5). Pre-blending, screw rotation speed, screw configuration, the number and length of kneading zones, temperature (°C) maintenance in different zones, and feed rate (g/min or kg/h) are important process variables impacting the granulation [28].
Temperature
Temperature plays a vital role in the melt granulation process. It affects the melting, mixing, and solidification of the drug and polymer. It is crucial to ensure that the processing temperature does not surpass the drug degradation temperature when utilizing melt granulation, particularly when the polymer Tg is greater than the drug degradation temperature. In such cases, employing a polymer with a lower Tg or incorporating a plasticizer can help decrease the processing temperature. However, extrusion in TSDG can be performed below the glass transition temperature or melting point of formulation components to maintain a dry state, as the process is supported by heat energy and shear applied during mixing [2, 25, 29]. Lower temperatures help maintain the crystallinity of the API during TSMG, and low melting point binders can help achieve this [30]. The temperature at which HME processing is carried out has a crucial role in the granulation process. When extrusion is performed at the melting temperature of the binder, it forms stronger and denser granules. This occurs because the amount of molten material increases, and the binding properties between particles are enhanced after cooling [31].
Screw Configuration
The number of screws and screw configuration will influence the degree of mixing (non-homogeneous blends), residence time, shear forces, and temperature distribution within the extruder [32]. An excess number of kneading zones may create denser granules (because of the high shear in the kneading zone) compared with a lower number of mixing zones. Also, it enhances the residence time of the blend in the extruder [33]. Adjusting conveying elements, such as increasing screw flight pitch, enhances granule output, reduces fines, and influences granule strength, while strategic placement after kneading zones minimizes lump formation, resulting in larger, denser granules with controllable sizes [31].
Screw Rotation Speed
Screw speed affects material residence time in the extruder. Faster speeds lead to shorter residence time but may increase the torque [30]. In TSG, screw speed has a minimal impact on granule size, showing only minor reductions, particularly noticeable with high-adhesive polymer blends [34].
Feed Rate
The material feed rate into the extruder directly affects the barrel fill level, indirectly affecting the residence time and the pressure applied to the granulation. The feeding rate significantly affects particle size and granule density/strength, with higher rates leading to larger, denser granules due to increased compressive forces, whereas lower feed rates lead to excessive fines. Therefore, controlling the feed rate allows for the optimization of granule properties [34, 35]. Moreover, maintaining a consistent and uniform feeding rate (maintaining the fill volume) to ensure reproducibility in granulation is a very important [30]. For TSWG, the essential parameters are binder solution flow rate (g/min or kg/h), spray nozzle design, binder distribution efficiency, and drying process [36].
The Gap Between Lab Scale and Commercial Scale Processing
TSG has significant potential as a continuous manufacturing and green technology in the pharmaceutical industry. Still, there are some gaps and challenges that exist in its commercialization. These gaps may vary based on the specific application and industry requirements. Despite the advancements in TSG, there still needs to be a deeper understanding of the underlying process mechanisms and the impact of process variables on granule properties. Developing robust models and control strategies for TSG is crucial to ensure consistent product quality and performance. Further research is needed to establish process-performance relationships, optimize process parameters, and enable real-time monitoring and control of the TSG process. Here are some common gaps in the commercialization of TSG, including process understanding and control, regulatory considerations, scale-up and manufacturing considerations (smaller batch size compared to conventional methods), equipment availability and cost, formulation complexity, and educational training [25].
Equipment Differences
Lab-scale TSG is typically performed using smaller equipment with lower capacity than commercial-scale production. The differences in equipment design, such as screw configuration, barrel length, and power, can affect the granulation process and result in variations in granule properties. Ensuring that the larger-scale twin screw granulator used in commercial production can achieve similar granulation outcomes as observed in the lab is essential. Pharmaceutical companies will focus on high-volume output with better quality in a shorter time. That can be achieved by modifying the TSG equipment. This may help to produce a larger quantity of granules for better output, but it should not show any variations in the granule properties. For example, TSG with multiple feeding zones and discharge ports (the barrel length should be the same as the lab scale) will produce larger quantities of granules with similar granule characteristics as the lab scale [28].
Mixing and Granulation Dynamics
Factors such as residence time, shear forces, and heat transfer may vary due to differences in equipment size and operating conditions. These variations can impact the granule characteristics, such as size distribution, flowability, and homogeneity. Process optimization studies and scale-up trials are necessary to understand and adjust for these differences.
Powder Flow and Feeding
The differences in powder flow can affect the uniformity of powder feeding and, consequently, the granule properties. Evaluating and optimizing powder flow and feeding systems during scale-up is essential to ensure consistent granulation outcomes.
Process Control and Parameters
Operating conditions, such as screw speed, torque, temperature, and feed rates, may need to be optimized for the larger-scale twin screw granulator. Identifying the critical process parameters and their impact on granule properties is crucial to maintaining consistent and reproducible results at the commercial scale. As TSG is a relatively new technology, there is a need for education and training programs to enhance the knowledge and skills of pharmaceutical scientists and manufacturing personnel. Adequate training on TSG principles, process optimization, and troubleshooting would help bridge the knowledge gap and promote the broader adoption of TSG in the industry. Addressing these gaps requires collaborative efforts between academia, industry, and regulatory bodies. Further research, technological advancements, and knowledge dissemination will contribute to the commercialization of TSG by addressing the challenges and providing comprehensive solutions for successful implementation at a larger scale [37].
Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing for Pharmaceutical Dosage Forms
Amid diverse applications of HME in pharmaceuticals, coupling it with fused deposition modeling (FDM) 3D printing technology has been widely explored in recent times in developing personalized and complex drug delivery systems. This new approach aims to move away from the traditional “one size fits all” dosage form concept in patient care and toward a more tailored and individualized treatment approach [38]. Therefore, conventional manufacturing techniques must transform to cater to patient-centric treatment with 3D fabricated personalized medication (Fig. 6).
FDM uses a drug-loaded thermoplastic polymer filament that passes through the nozzle of the printer, where it melts and deposits onto the surface to obtain a desired 3D structure, as shown in Fig. 6. The most efficient way to achieve high drug loading of filaments is through HME, where a mixture of thermally stable drug, polymer, and plasticizer (if needed) are processed (undergoes mechanical and thermal stress) to draw a filament [39]. The polymers with lower glass transition temperature (Tg) than the melting point (Tm) of the drug are usually preferred to avoid thermal degradation of the drug during the processing. Some of the most commonly used pharmaceutical-grade thermoplastic polymers for producing filaments for FDM are Eudragits, Polyvinyl pyrrolidone (PVP), HPC, Polyethylene glycol (PEG), etc. [40].
To achieve the desired precision and print quality of dosage forms, it is crucial to identify and optimize the critical process parameters (CPPs) of both the HME and the FDM printer during fabrication. The material’s melting temperatures often rise above the barrel temperature due to the mechanical shear caused during mixing and extrusion, which leads to unwanted degradation. To overcome this, plasticizers (polyethylene glycol (PEG), poloxamers (188 and 407), triethyl citrate (TEC), etc.) are used, which aid in processing extrusion at low temperatures as it reduces Tm of the drug, Tg of polymers, and additionally the melt viscosity of materials, thus reducing the torque [41]. Despite these advantages of plasticizers in filament fabrication, they impart good flexibility to filaments, making them suitable for processing through FDM.
There are several characterization tests to determine the suitability of filaments for FDM 3D printing, which entail the three-point bend test (brittleness, Young’s modulus, and distance at break), Repka-Zhang test (brittleness/stiffness), Hooke’s law (constant “K”), and FDM feedability testing (flexibility).
Moving forward, the ones with good filament properties and suitability are processed through an FDM 3D printer. The main CPPs affecting some of the quality attributes (weight, dimensional authenticity, and overall print reproducibility) of dosage forms are printing speed, nozzle temperature, layer height, and platform temperature [42]. The weight of the dosage form is affected by the nozzle temperature and printing speed. The weight decreases with increasing the printing speed because of the voids created due to improper melting of filament, while weight increases with increasing the nozzle temperature. The uniform diameter of the filament plays a key role in maintaining constant weight and achieving good printability. However, printing speed has more influence on weight than the nozzle temperature. Higher printing speeds lead to the low resolution of the object with poor print quality [42, 43]. With the decrease in nozzle temperature, layer height increases, which in turn leads to poor resolution. The nozzle temperature must be optimum enough to achieve the required melt viscosity of filament for the molten mass to spread on the build platform with uniform layer height. Having a strong understanding of filament’s melt rheology is important as the viscosity of molten mass affects printability through FDM. Typically, the build platform’s temperature is set at 60–70 °C for easy removal of the printed object [43].
Overall, HME-coupled FDM 3D printing made possible several advancements in developing novel pharmaceutical dosage forms, some of which are listed in Table II. However, certain limitations of FDM 3D printing, including the biodegradable polymer availability, low printing resolution, and possibilities of drug degradation, need to be addressed [44].
Drug Complexes/Solid Dispersions via Hot-Melt Extrusion
Cyclodextrin Complexes
For many years, the pharmaceutical industry has extensively researched and used cyclodextrins (CDs) to increase the solubility of poorly aqueous soluble APIs. CDs are toroidal-shaped cyclic oligosaccharide molecules with a hydrophilic outer surface and a hydrophobic core. Drug molecules and CDs interact to form host–guest complexes, where the hydrophobic cavity of the CDs (host molecule) provides an advantage for the entrapment of poorly aqueous soluble APIs (guest molecule). Since the API and CD interact at the molecular level, the stoichiometry of molecules at which these two components interact must be determined priorly, which can be done using various in-silico (molecular docking) and in-vitro (phase solubility studies or Job's plot) methods. In addition to improving the solubility of API, the formation of complexes enhances the stability (such as photo and thermal stability) and palatability of drugs [45]. Both the API and CD molecules must be able to interact freely to form the complex, which is impossible when they exist in the solid state. The API and CDs are typically dissolved in a common solvent or solvent mixture to create the ideal conditions for the API and CD interaction. However, due to the lack of solvent usage in HME, the conditions for the drug-cyclodextrin complexation during the extrusion process are difficult to achieve. As a result, various alternative approaches have been investigated, such as adding solvent to HME to form the complex (hot-liquid extrusion (HLE)) or adding a polymer. In HLE, the liquid aids in the formation of a complex between API and CD. The principle behind this technique may be like that of the kneading technique, in which the physical mixture is intensively mixed with the help of liquid. However, the intensity, consistency, and uniformity of mixing in HLE are far superior to the kneading technique, resulting in a greater efficiency of complexation between the host and guest molecules. This technique was utilized by Manne et al. to form Carbamazepine-CD complexes, and they studied the effect of solvent (water and aqueous buffer), screw speed, temperature, and molar ratio of complexation between the two reactants. The solvent systems that kept the API in a unionized state (high hydrophobicity) resulted in high complexation efficiencies resulting in better improvement of solubility. The temperature has little effect on complexation because the interaction between the host and guest molecules involves the guest molecules’ replacement of water molecules from the host cavity. However, the complexation efficiency at near room temperature was higher than at elevated temperatures. The screw speed is indirectly proportional to residence time; therefore, the optimum speed should be chosen by potentially eliminating the detrimental screw speeds, which vary depending on the solvent system, temperature, and nature of the selected API and CD [46].
In an alternative method, a third component, typically a polymer, is added to aid inclusion complexation in HME, transforming the binary inclusion complex system (host and guest) into a ternary inclusion complex system [47]. This method has been reported in various studies to increase the solubility of API using CD through HME. The choice of polymer is critical because the added polymer must dissolve the drug or be miscible with the molten phase for the drug to be included in the CD’s hydrophobic cavity. In this procedure, there are three main parameters that need to be considered before extrusion through HME, that are rheology of molten polymer and drug, melting point of drug, and drug-polymer miscibility. Since CD is a non-thermoplastic component of the formulation, the rheology of the molten polymer and drug is critical and must provide adequate lubrication during the extrusion process, which also serves as a deterministic factor in deciding the temperature and polymer percentage to be used in the formulation [48]. The rheological behavior of molten components can be determined by using a frequency sweep test, typically performed above the melting (Tm) and glass temperatures (Tg) of the API and polymer, respectively [49]. On the other hand, the API’s melting point is also a deciding factor in polymer selection; the drug must be either in a molten state or dissolved in the molten polymer for complexation to occur. Polymer miscibility is not critical for APIs with low melting temperatures since the molten API can either be miscible or dispersed with the molten polymer, forming inclusion complexes and suitable extrusion conditions [50]. If the drug has a high melting temperature, the polymer’s miscibility and solubility become a critical factor, and the polymer and its percentage in the formulation should be enough to solubilize high proportions of API, creating favorable processing conditions for complex formation [51]. The screw speed should be chosen to maintain the least amount of torque while also ensuring the thermal stability of the formulation components [50].
Drug-Resin Complexes
Drug-resin complexes (DRCs) are generated through an ion-exchange reaction between drugs and Ion-Exchange resins (IERs), forming dynamic supramolecular complexes. IERs are high molecular weight and highly cross-linked polymeric structures, existing as insoluble salts in water but capable of dissociating and releasing ions when exposed to water. During the formation of DRCs, IERs can exchange ions with high-affinity ions, which, in this case, are drugs. These IERs can possess either cationic or anionic properties, and the strength of ion exchange relies on the affinity of their functional groups [52]. However, for the ion exchange process to occur between the IER and the drug, the drug needs to be in its salt form, preferably as hydrochloride or hydrobromide. DRCs offer numerous advantages, such as taste masking, enhanced stability, and rapid dissolution. Additionally, by coating the DRC particles with suitable polymers, extended-release and abuse-deterrent formulations can also be developed [53]. Traditionally, DRCs are prepared using a conventional batch process that often necessitates large quantities of solvent. To address this concern, researchers have been exploring the hot melt extrusion method. This approach aims to reduce solvent requirements. To date, only two studies have reported the use of extrusion processes to produce DRCs, one utilizing hot melt extrusion and the other incorporating water as a solvent during extrusion to facilitate complex formation.
Tan et al. utilized the hot melt extrusion technique to prepare DRCs of quinine hydrochloride. Two weak cationic resins, namely Amberlite™ IRP88 (IRP88) and Amberlite™ IRP64 (IRP64), containing carboxylate and carboxyl groups, respectively, were evaluated. The research findings highlighted the significant influence of the drug-to-resin ratio and extrusion temperature on DRC formation. Lower drug-to-IER ratios resulted in higher complexation efficiency, presumably due to the increased availability of binding sites. The extrusion temperature played a crucial role in complex formation since the absence of an aqueous solvent made it challenging to generate ions, which are essential for complexation. Therefore, higher temperatures were required to ionize the drug and IER, while also reducing the viscosity of the molten material during extrusion, facilitating ion diffusion and complex formation. The screw speed exhibited limited impact on the DRC formulation, necessitating optimization to ensure a smooth extrusion process without hindrances. The drug’s affinity for the resin was a critical factor in resin selection and required screening. In this study, IRP88 demonstrated superior performance over IRP64 in forming DRCs of quinine hydrochloride, as IRP64 failed to diminish the peak intensities at 2500–2600 cm−1 in the infrared spectrum of quinine hydrochloride. Dissolution studies revealed the rapid dissolution of quinine hydrochloride from the DRCs [54].
In a separate study, water was employed as an assisting agent for DRC formation in the hot melt extrusion (HME) process. The preparation involved pre-blending the drug and resin and extruding using a 16-mm twin screw extruder. The pre-blend was introduced through the first port (feed port), while de-ionized water was introduced through the second port to generate a thick suspension. Subsequently, the thick suspension was dried in an oven, forming a dried DRC. The dried DRC was then subjected to HME along with thermoplastic polymers to produce a tamper-resistant formulation [55].
Solid Dispersions
HME has been successfully employed for the formation of solid dispersions to improve various properties of the drug, like solubility and bioavailability. These solid dispersions are formed when the API is dispersed uniformly in the polymeric or lipidic carrier matrices. These are further divided into various categories, based on the molecular state of dispersed API in the carrier: crystalline solid dispersions (CSD) and amorphous solid dispersions (ASD) [56]. In ASDs, the drug is present in amorphous nature with a large surface area, thereby improving the drug’s solubility. The amorphous nature is a high energy thermodynamically unstable state, which tends to convert into a more thermodynamically stable crystalline state, thus affecting the stability of the formulation. In contrast, CSDs consist of the microcrystalline drug (i.e., reduced particle size), which is in a more stable state and offers more stability of dosage form while compromising the solubility of the drug [56, 57].
Amorphous Solid Dispersions
During the preparation of ASDs in HME, both the polymer and drug undergo melting inside the barrel and are mixed thoroughly by the rotating screws. Which was then extruded out as granules or as a filament. These filaments can be used to print dosage forms using FDM 3D printing technology, or the extrudates can be processed to form granules or milled and further processed to form tablets or capsules [58]. The temperature, along with vigorous mixing inside the barrel, melts the drug or reduces the particle size of the drug and allows it to be miscible or dissolve within the molten polymer, thereby improving the content uniformity and stability of the drug’s amorphous nature [59]. Therefore, proper selection of polymer carriers is of utmost importance in the successful development of ASD since they account for a large portion comprising about 50 to 90% w/w of ASD and are responsible for limiting the molecular mobility of the drug and stabilizing the ASD. Usually, hydrophilic thermoplastic polymers are used for the preparation of ASD. Therefore, various factors of polymer need to be considered, i.e., molecular weight, hygroscopicity, Tg, degradation temperature, and chemical reactivity with the drug, etc. [60].
The molecular weight of the polymer directly influences intrinsic viscosity, which subsequently affects the drug dissolution and the formulation's physical stability [61, 62]. A study conducted by Auch et al. to assess the impact of molecular weight on the dissolution of ASD manufactured using HME revealed that an increase in the molecular weight of the polymer decreased the dissolution of the ASD [61]. Mohapatra et al. investigated the effect of molecular weight on the physical stability of ASD, they discovered that increasing the molecular weight of the polymer reduces the drug's molecular mobility, preventing crystallization [62]. The hygroscopicity of the polymer influences the overall stability of the formulation in addition to drug crystallization. Low hygroscopic polymers are always preferred for the preparation of ASD [60]. The Tg of the polymer used in the preparation of ASD is in the range of 50–200 °C. The Tg of the polymer and the miscibility of the drug in the polymer are correlated. The drug can either be miscible (below or above the Tg of polymer) or immiscible, which corresponds to the stability of the formed ASD in the order of miscibility: below Tg of polymer (most stable) > above Tg of polymer > immiscible within the polymer (least stable) [63]. While, in the case of the chemical reactivity of polymer with drug, the reactivity through covalent bond formation is not desirable since it changes the characteristics of the drug molecule. However, the non-covalent bonds (H-bond, hydrophobic interactions, etc.) formed between API and polymers reduce the molecular mobility of API within the polymer matrix and hinder the recrystallization.
Crystalline Solid Dispersions
In contrast to ASD, during the preparation of CSDs, the crystalline nature of the API is preserved. These are primarily used to avoid the stability issues associated with API crystallinity, and they can also accommodate higher drug-loading capacities than ASDs. For the preparation of CSDs, a temperature below the melting point of API is used for HME processing. Polymers with lower Tg than Tm of API are utilized. In a study conducted by Prasad et al., CSDs of mefenamic acid were developed. Wherein, the temperatures utilized are lower than the Tm of the drug, and a plasticizer was used to further reduce the processing temperatures in HME. The DSC and XRD results indicate the retaining of the crystalline nature of API after extrusion especially when the drug loading was above the 20% w/w. These results indicate the criticalness of drug loading in the formulation. Since the drug can be miscible in the liquid polymer to an extent, the higher quantities of the drug above the saturation level in the liquid polymer are essential for the preparation of CSD [64]. In an alternative approach to preparing CSDs, a hydrophilic solid crystalline carrier is utilized instead of a polymer. In this method, known as solid crystal suspensions (SCSs), the crystalline drug is ground and dispersed within the solid crystalline carrier. Unlike in CSDs, there is no direct interaction between the drug and the carrier in SCSs; however, a thermodynamically stable system is achieved. In the hot melt extrusion (HME) process, the crystalline carrier melts and suspends the crystalline drug within it. During extrusion, the drug may either melt and then recrystallize back into its crystalline state as the temperature decreases, or the drug may remain solid while undergoing particle size reduction and dispersal within the carrier. Upon dissolution, the hydrophilic carrier rapidly dissolves, exposing the drug particles that have been reduced in size, thereby facilitating faster dissolution [65].
Reitz et al. conducted a study to investigate the impact of process parameters on the development of griseofulvin SCSs. They employed mannitol as the solid carrier for the SCSs and identified barrel temperature and screw speed as critical process parameters, studying their effects on drug particle size reduction, dissolution, and porosity. The results indicated that temperatures above the melting point of mannitol exhibited favorable processability, while temperatures below its melting point subjected the barrel to significant stress. Higher screw speeds led to a reduction in the particle size of griseofulvin but had no significant impact on dissolution. Therefore, temperatures above the melting point of the solid carrier and lower screw speeds were deemed essential for the successful development of SCSs. Consequently, it can be concluded that the enhancement of dissolution in SCSs is predominantly influenced by the rate of particle size reduction rather than porosity. However, no correlation was established between the process parameters and porosity in this study [66].
Cocrystals
The preparation of cocrystals is one of the promising approaches to enhance the solubility and, thus, bioavailability of poorly soluble drugs. Besides, cocrystals alter physicochemical properties, improving stability and permeability and masking the bitter taste of drug substances without significantly affecting their pharmacological activity. Cocrystals are multi-component systems in which the active pharmaceutical ingredient (API) and coformer excipient/another API in a definite stoichiometric ratio are bonded together via either hydrogen bonds, van der Waals forces, or π-π interactions. Compounds with functional groups such as carboxylic acids, alcohols, and amides can produce cocrystals. Typically, compounds containing heteromolecular synthons such as carboxylic acid-pyridine, carboxylic acid-amide, and alcohol-pyridine are more favorable to forming cocrystals than homosynthons (carboxylic acid/amide dimers) [67, 68].
Cocrystals preparation methods were broadly categorized as solution-based and solid-state methods. These methods include solvent evaporation, antisolvent, reaction crystallization, solid-state grinding, and hot-melt extrusion. Solid-state methods are preferred over solution-based methods due to the no or minimal use of solvent, which imparts better stability to cocrystals [68, 69]. HME is one of the most convenient cocrystal preparation techniques and offers efficient, homogenous mixing of molten components in the heated barrel without the involvement of a solvent [70, 71]. Besides greener technology, the ease of the scale-up process and the stability of products with HME make this an industry-feasible technique to prepare cocrystals. In addition, this technology potentially produces high-quality cocrystals with effective cocrystallization of API. Until now, hot-melt extruded cocrystals were prepared by two methodologies. First, the drug and coformer were extruded; second, matrix-assisted cocrystallization, in which the polymer enhances the cocrystallization process [72, 73].
When preparing cocrystals using the hot-melt extrusion technique, it is important to pay attention to the following critical quality attributes (CQAs).
Composition
The composition of the cocrystal, including the ratio and identity of the components, is critical to the formation and stability of the cocrystal. Therefore, the composition should be carefully controlled to ensure that the desired cocrystal is formed and that the product has the required physical and chemical properties [74]. Furthermore, the aqueous solubility of the coformer is equally important to enhance the solubility of the cocrystals [75].
Purity
The purity of the starting materials used in the HME process is critical to the quality of the final product. Impurities can affect the formation and stability of the cocrystal and the product's safety and efficacy.
Particle Size and Distribution
The particle size and distribution of the cocrystal particles produced by HME are important CQAs as they can impact the dissolution and bioavailability of the product. It is vital to control the size and distribution of the particles to ensure consistent product performance [76, 77].
Thermal Properties
The thermal properties of the cocrystal, such as the melting point and glass transition temperature, are critical to the processing and storage of the product. Therefore, these properties should be controlled to warrant that the product can be processed using HME and remains stable during storage.
HME Process Parameters
The process parameters, such as extruder type, temperature, screw speed, screw configuration, and feed rate, can also affect the properties of the final product and should be optimized accordingly [71, 78]. Twin-screw extruder efficiently mixes the components leading to high-quality and homogenous cocrystals over a single-screw extruder. Processing temperature is critical when preparing cocrystals, as it affects the viscosity, material residence time in the barrel, and co-crystallization. Complete cocrystallization occurs when the HME process is performed at or near the cocrystal melting temperature. Similarly, the lower screw speed allows the material to reside in the barrel for a long, permitting effective molecular interactions between the API and coformer owing to the high-quality cocrystal [72]. At the same time, the quality of a product is determined by the degree of mixing required, which is primarily influenced by the L/D ratio. To achieve the most effective mixing, the screw configuration should be able to incorporate the components which create intense shear forces. This will result in a longer material residence time in the extruder, leading to better mixing and significantly improving the quality of cocrystals. For the successful creation of cocrystals through extrusion, it is crucial to maintain an optimal feed rate. If the feed rate is too high, the drug-coformer mixture may not have sufficient time to pass through the extruder. This can lead to increased torque and pressure, ultimately blocking the extruder nozzle and a failed process [34, 70, 71]. However, all the HME process parameters need to be optimized case-to-case.
Overall, controlling these critical quality attributes is crucial for successfully preparing cocrystals using HME to ensure the quality and consistency of the product.
HME Scale-up Challenges, Considerations, and Models
HME/TSE Equipment Sizes and Scale-up Challenges
Hot-melt extrusion has become a widely used technique to enhance the solubility of pharmaceutical products. Extruder manufacturers such as Leistritz and Thermofisher have been continuously developing smaller equipment to meet the pharma industry’s demand. Smaller-scale lab equipment is necessary to conduct formulation and product development studies with limited API available in early-stage development. However, it is crucial to have a seamless scale-up from lab to production level. This ensures that the product developed in the lab can be produced on a larger scale without any issues. Manufacturers of TSEs offer various sizes of twin-screw extruders. Clients can choose the size that best suits their needs (Table III).
To scale up pilot production for HME, the process can be run longer, or material feed rate and screw speed can be increased up to maximum equipment throughput. For commercial manufacturing, lab or pilot-scale extruders need more throughput. To achieve larger scales, increase equipment size to meet commercial demand. Scaling up a manufacturing process can be challenging. Simply increasing the throughput or feed rate can affect the product quality. Changing the screw speed or feed rate can cause machine overload and affect the product quality. Similarly, increasing the barrel size without adjusting the feeding rate can impact product quality. In continuous manufacturing, transferring from a laboratory to a production level is complex due to several unit operations combined within a single apparatus.
The laboratory extruder’s fundamental geometry must match that of the larger extruder. One of the key parameters to consider is the ratio of the outer and inner diameter of the screw. Modular and exchangeable TSE screw elements play a crucial role in achieving a homogenous product. During scale-up, it is critical to maintain a similar screw profile. When using larger equipment, there may be limitations on mass or heat transfer that can impact product uniformity. As screw diameter increases in larger equipment, the tip speed of the rotating screw flights also increases, leading to a higher peak shear to which the material is exposed. Design-of-experiment (DoE) approaches, process models, and software can be useful to define and predict the process-based design space.
Despite the challenges and difficulties involved, it is possible to successfully optimize the scale-up of HME processes by having a good knowledge of formulation and carefully evaluating the impact of critical process parameters (CPP) and their boundary conditions on the critical quality attributes (CQA) during product development. It is more predictable, reproducible, and consistent to scale up from a lab scale to a larger TSE with a similar geometry than a batch process scale-up. To ensure a successful design of the scale-up in HME processing, it is essential to have a good understanding and management of several parameters such as screw design, degree of screw fill, screw speed, feed rate, residence time distribution, processing temperature profile, specific mechanical energy consumption, and their influence on product quality [79,80,81,82].
Scale-up Models and Formulas in HME
Several case studies have been reported in the literature, and the following are the most proposed scale-up models [81, 82]. Corresponding formulas or the model is given in Table IV.
-
Adiabatic melt extrusion process derived from the cubic law: Based on these considerations, adiabatic extrusion processes can be scaled up with Eq. (1), in the case that sufficient energy is provided by viscous dissipation at ideally no heat loss or gain from the surrounding environment. The output will scale by the diameter ratio to the power of three (q = 3) for extruders with geometrical similarity and processes running at the same rotation speed. Similarly, a square law (q = 2) can also be suggested based on the heat transfer when considering a successful scale-up. Nevertheless, the balance can deviate based on the geometric similarity and the melt temperature because they might not be constant throughout the scale-up.
-
Volumetric scale-up: In the continuous manufacturing (CM) of pharmaceutical products using HME processes is applied to maintain the same degree of fill and the same RTD. For geometrically similar extruders with different diameters, the final feed rate in a twin-screw extruder is assessed using Eq. (2). In simplified conditions, if volumetric scale-up is conducted using similar geometric extruders (e.g., 11-mm or 16-mm twin-screw extruder from Thermo Fisher, Germany) and the screw speed is kept constant, the process throughput is expected to obey the cubic law.
-
Heat Transfer Rate: Scale-up of the CM of pharmaceuticals using HME processes can be assessed by optimizing the heat transfer of the barrel, where the scale-up of the manufacturing will entirely be dependent on the heat transfer. In this method, the surface area for heat transfer is taken to be equivalent to the barrel surface area. Based on this, the process throughput will be scaled up while maintaining the heat transfer rate, as shown by Eq. (3).
-
Specific torque and specific energy (SE) input is considered a critical parameter in the production of high-energy compositions, including amorphous systems. Therefore, successful scale-up of a CM process via HME depends largely on the steady constant level of SE input. Generally, the mechanical energy input in an HME process during the optimization of scale-up methods is calculated and determined by using Eq. (4).
-
Specific mechanical energy (SME) measures the total mechanical energy (as a form of heat) put into the extrudate during high shear mixing. Specific mechanical energy consumption (SMEC) represents the energy entering the extrusion system per unit mass through viscous dissipation. As shown by Eq. (5), the SMEC depends on the torque, rotation of the screw, and the feed rate or throughput, and is measured in kJ/kg.
-
Shear rate: In a TSE system, shear forces result in mixing and, thus, the shear rate determines the velocity gradient between two surfaces moving at different speeds. In a typical TSE, the shear rate is calculated as a function of screw outside diameter, overflight gap, and the screw speed, as shown by Eq. (6).
-
Shear stress is referred to as the magnitude of the applied stress inside the barrel that the conveying materials experience and is expressed by Eq. (7) as a function of the shear rate and viscosity. Barrel temperatures have a key role in controlling the viscosity of the melt inside a barrel. Low or high viscosity impacts the mixing quality (e.g., high viscosity facilitates dispersive mixing).
-
Residence time distribution is highly dependent upon the degree of screw fill. Equation (8) is used to determine the residence time.
HME as a Continuous Manufacturing Process
CM is a process of producing products by continuously feeding input materials, processing them, and simultaneously removing the output material from the system without any interruptions. In this method, the materials remain in constant motion while subjected to mechanical or heat treatment, resulting in chemical or physical transformation. Continuous processing is widely used in the chemical, fertilizer, and petrochemical industries. However, it is still a relatively new concept in the pharmaceutical industry. About three decades ago, Fernando Muzzio from Rutgers University initiated the first research program on the pharmaceutical continuous manufacturing [83].
Many pharmaceutical manufacturing processes, such as tablet compression, encapsulation, roller compaction, and extrusion, are naturally continuous, yet they are often used in isolation as batch processing equipment. Traditional batch manufacturing processes follow a sequential approach where the material is introduced into a specific unit operation and transformed into an intermediate product, which is then discharged and tested offline. The intermediate product is then transported to the next unit operation for further processing. The holding time for intermediates can vary depending on the scale, which requires hold time studies. These steps are usually repeated over multiple unit operations, for instance, blending, hot melt extrusion, milling, compression, and coating (Fig. 7). On the other hand, the same unit operations can be integrated via continuous manufacturing (CM). In this process, the material is automatically transferred, monitored, and controlled in-line along the manufacturing path. The starting material is continuously charged into the first process unit at the beginning of the line, while the final product is discharged at the end of the process unit. The twin-screw extruder can be integrated with downstream tablet manufacturing unit operations continuously to set up a true end-to-end continuous solid oral dosage form manufacturing process (Fig. 8). Process analytical technology (PAT) can establish a control strategy (feed-back or feed-forward). In such a continuous process, the time to make a final product takes less without needing intermediate testing and hold time studies [84].
PAT Considerations
PAT is a real-time process analytics and data analysis method that encourages a risk-based approach in pharmaceutical processes, which complies with the ICH Q9 guideline (Quality Risk Management). PAT enhances the comprehension and management of production processes and enables real-time final product testing and release. A wide range of sensor technologies are available in PAT, which can be univariate or multivariate and used in-line or online. The sensor’s location at the testing point must be carefully evaluated to obtain accurate information. For instance, HME constitutes a high-pressure and high-temperature process. Therefore, the selection of PAT technology and point of installation must consider these factors [85].
Type of PAT Sensors
The most common PAT sensors used in CM are listed below [84, 86].
Near-infrared spectroscopy is a light absorption spectroscopic technique. In this, the sample is irradiated with light in the 1000–2500 nm wavelength range. Sample molecules absorb light photons of specific wavelengths and get excited to a higher vibrational state, resulting in a dipole moment change. The molecular structure can be evaluated depending on the specific wavelength absorbed and the spectrum created. Polar bonds and bonds with larger differences in atomic mass (-CH, -OH, and -NH bonds) show shorter absorption of the NIR radiation. In HME, the spectrum changes depending on the formulation and processing conditions (such as screw speed, screw design, feed rate, temperature profile) that affect product temperature and pressure. Using robust models, API content and API-polymer interaction could be evaluated at the die [85].
Raman spectroscopy is based on the measurement of frequency shifts in reflected light by molecules compared to incident monochromatic laser light. Inelastic scattering of photons by a molecule with induced transition between vibrational states of the irradiated molecules results in frequency shifts. Then, 785 nm diodes or 1064 nm ND:YAG lasers are the most commonly used. Raman is well suited for non-polar bonds (C–C, C = C, S–S, N = N) in carbon chains and aromatic rings. The spectra are not affected by the presence of water [85].
Chemical Imaging utilizes two-dimensional spatially resolved information using Raman and NIR sensors simultaneously. It provides information about the chemical composition of a sample [87].
Particle size analysis is used in CM to collect information on particle size distribution and sphericity of particles. Techniques such as image analysis, focused beam, laser diffraction, or filter velocimetry could be employed [88].
Approved Drug Products Manufactured by CM
In 2015, the first CM solid oral dose product approved by the FDA and EMA was Orkambi®, which was made by Vertex for Cystic Fibrosis indication. Table V shows a list of approved drug products that are manufactured by continuous process [89]. Many CM products are in the pipeline, and more of them are expected to be approved in the next few years.
Advantages of Pharmaceutical Continuous Manufacturing
CM has several advantages including quality, regulation, sustainability, and economics (see Fig. 9). It is a true representation of quality by design (QbD) by building quality into the product instead of relying on final product testing. By implementing inline in-process monitoring and control systems, endpoint testing and complete batch rejection can be avoided. This reduces the need for expensive and time-consuming testing, batch rejection, and QA management efforts, thus reducing the cost of goods. Continuous pharmaceutical manufacturing is cost-efficient and flexible. It enables real-time monitoring and control of critical quality attributes (CQAs) and critical process parameters (CPPs) based on the quality by design (QbD) concept. This allows for real-time quality assurance, known as real-time release (RTR). CM has led to a new quality assurance concept, quality by control (QbC), that involves designing a robust manufacturing system and an active process control system based on a high degree of quantitative and predictive product and process understanding. QbC enables reliable batch and continuous process operations and real-time release of pharmaceutical products. Pharmaceutical CM offers efficiency and flexibility advantages, with shorter supply chain time and improved security and responsiveness. It is a sustainable technology, solvent-free with lower energy consumption. The small footprint and modular unit operations make it an attractive technology over conventional batch processes.
Investing in CM technology in the USA is more economical for brand and generic companies reported by Clifford V. Rossi in 2022. This study compared continuous (CM) and conventional batch manufacturing for oral solid dosage pharmaceuticals (OSD) production. The simulation analysis showed that investing in batch technology is more profitable. Continuous manufacturing could make OSD pharmaceuticals more economically attractive in the USA [90, 91].
Oral Solid Dosage Continuous Manufacturing Challenges
Despite the benefits of continuous manufacturing in developing oral solid dosage forms, it also presents several challenges. One of the main difficulties continuous production systems face today is accurately measuring the starting materials. These systems must constantly measure out both active ingredients and excipients at a consistent mass flow rate. However, all current dosing systems experience fluctuations in their mass flow rate over time. As a result, it is necessary to use PAT tools to verify the API content online. Twin-screw granulation in continuous processes can cause particle-size segregation due to changes in granule density and bimodal particle-size distribution. This can negatively impact tablet properties, so one should consider this while using these technologies. In addition, the challenges associated with scale-up and regulatory considerations were discussed in the respective sections. Continuous manufacturing offers numerous benefits in terms of efficiency, quality, and cost-effectiveness, which outweigh the challenges involved. However, it is essential to collaborate with industry stakeholders, conduct ongoing research and development, and commit to implementing best practices to overcome these challenges effectively.
Regulatory Considerations
Adopting innovative approaches to pharmaceutical manufacturing may present both technical and regulatory challenges. Pharmaceutical companies may have concerns that introducing CM, due to its novel nature, could result in regulatory challenges and approval delays. The FDA is cognizant of these challenges and has created the Emerging Technologies Program (ETP) in the CDER’s Office of Pharmaceutical Quality in 2014. ETP is a collaborative program where industry representatives and Emerging Technology Team (ETT) members can meet before regulatory filing to discuss, identify, and resolve potential technical and regulatory issues. ETT members are representatives from all relevant FDA quality review and inspection programs, including the Office of Pharmaceutical Quality (OPQ), CDER’s Office of Compliance, and the regulatory office [92]. As of August 2022, ETT has accepted 46 Continuous manufacturing proposals and, as of March 2023, approved 14 CM applications.
CM applicants had relatively shorter times to approval and market as compared to similar batch applications, based on the mean or median times to approval (8 or 3 months faster) and marketing (12 or 4 months faster) from submission, translating to an estimated $171–537 M in early revenue benefit [93]. July 10, 2023, ICH guideline Q13 on continuous manufacturing of drug substances and drug products has come into effect. This guideline describes scientific and regulatory considerations for the development, implementation, operation, and lifecycle management of CM. The regulatory environment for PCM is very conducive, and it is expected to gain more traction in the next few years [94].
Marketed Products
Hot-melt extrusion has revolutionized the production of various marketed products across industries (Table VI). In the pharmaceutical sector, it is extensively utilized to fabricate innovative drug delivery systems, with enhanced solubility and bioavailability. The robustness of this technology makes continuous manufacturing a reality. Additionally, the technology is exploited in the manufacturing of 3D printing filaments, demonstrating its versatility and impact in bringing diverse and tailored products to the market.
Conclusion
Twin screw extrusion technology has completely transformed from an adapted technology from the plastic’s industry into pharmaceutical manufacturing. It has gained wide interest from the pharmaceutical industry as it permits the persistent development of dosage forms for various applications with few equipment and process modifications. Twin screw extrusion technology entails a small equipment footprint, is a solvent-free process that is easy to scale up as well as capable to integrate all the unit operations in single step making it suitable for continuous manufacturing processes. As a result of innovative TSE applications as well as extensive research in scaling up the process, there are an increasing number of approved HME products in the market now. These authors expect this trend to advance and continue.
References
Koutsamanis I, Roblegg E, Spoerk M. Controlled delivery via hot-melt extrusion: a focus on non-biodegradable carriers for non-oral applications. Journal of Drug Delivery Science and Technology. 2023;81:104289.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33:909–26.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev Ind Pharm. 2007;33:1043–57.
Kallakunta VR, Sarabu S, Bandari S, Tiwari R, Patil H, Repka MA. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part I. Expert Opin Drug Deliv. 2019;16:539–50.
Sarabu S, Bandari S, Kallakunta VR, Tiwari R, Patil H, Repka MA. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part II. Expert Opin Drug Deliv. 2019;16:567–82.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17:20–42.
Smyth HDC, Hickey AJ. Carriers in drug powder delivery. Am J Drug Deliv. 2005;3:117–32.
Spahn JE, Hefnawy A, Smyth HDC, Zhang F. Development of a novel method for the continuous blending of carrier-based dry powders for inhalation using a co-rotating twin-screw extruder. Int J Pharm. 2022;623:121914.
Ren A, Koleng JJ, Costello M, Spahn JE, Smyth HDC, Zhang F. Twin-screw continuous mixing can produce dry powder inhalation mixtures for pulmonary delivery. J Pharm Sci. 2023;112:272–81.
Ren A, Spahn JE, Smyth HDC, Zhang F. Twin-screw continuous mixing: Effect of mixing elements and processing parameters on properties of aerosol powders. Powder Technol. 2023;424:118519.
Martin C. Twin screw extruders as continuous mixers for thermal processing: a technical and historical perspective. AAPS PharmSciTech. 2016;17:3–19.
Pilcer G, Wauthoz N, Amighi K. Lactose characteristics and the generation of the aerosol. Adv Drug Deliv Rev. 2012;64:233–56.
Schmidt A, de Waard H, Moll K-P, Kleinebudde P, Krumme M. Simplified end-to-end continuous manufacturing by feeding API suspensions in twin-screw wet granulation. Eur J Pharm Biopharm. 2018;133:224–31.
Hare C, Ghadiri M, Dennehy R. Prediction of attrition in agitated particle beds. Chem Eng Sci. 2011;66:4757–70.
Meena AK, Desai D, Serajuddin ATM. Development and optimization of a wet granulation process at elevated temperature for a poorly compactible drug using twin screw extruder for continuous manufacturing. J Pharm Sci. 2017;106:589–600.
Schmidt A, de Waard H, Kleinebudde P, Krumme M. Continuous single-step wet granulation with integrated in-barrel-drying. Pharm Res. 2018;35:167.
Kreimer M, Aigner I, Lepek D, Khinast J. Continuous drying of pharmaceutical powders using a twin-screw extruder. Org process Res Dev. 2018;22:813–23.
Haser A, Kittikunakorn N, Dippold E, DiNunzio JC, Blincoe W. Continuous twin-screw wet granulation process with in-barrel drying and NIR setup for real-time moisture monitoring. Int J Pharm. 2023;630: 122377.
Li M, Ioannidis N, Gogos C, Bilgili E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur J Pharm Biopharm. 2017;119:68–80.
Khinast J, Baumgartner R, Roblegg E. Nano-extrusion: a one-step process for manufacturing of solid nanoparticle formulations directly from the liquid phase. AAPS PharmSciTech. 2013;14:601–4.
Ye X, Patil H, Feng X, Tiwari RV, Lu J, Gryczke A, et al. Conjugation of hot-melt extrusion with high-pressure homogenization: a novel method of continuously preparing nanocrystal solid dispersions. AAPS PharmSciTech. 2016;17:78–88.
Baumgartner R, Eitzlmayr A, Matsko N, Tetyczka C, Khinast J, Roblegg E. Nano-extrusion: a promising tool for continuous manufacturing of solid nano-formulations. Int J Pharm. 2014;477:1–11.
Li M, Furey C, Skros J, Xu O, Rahman M, Azad M, et al. Impact of matrix surface area on Griseofulvin release from extrudates prepared via nanoextrusion. Pharmaceutics. 2021;13:1036.
Nyavanandi D, Mandati P, Narala S, Alzahrani A, Kolimi P, Vemula SK, et al. Twin screw melt granulation: a single step approach for developing self-emulsifying drug delivery system for lipophilic drugs. Pharmaceutics. 2023;15:2267.
Bandari S, Nyavanandi D, Kallakunta VR, Janga KY, Sarabu S, Butreddy A, et al. Continuous twin screw granulation—an advanced alternative granulation technology for use in the pharmaceutical industry. Int J Pharm. 2020;580: 119215.
Vercruysse J, Burggraeve A, Fonteyne M, Cappuyns P, Delaet U, Van Assche I, et al. Impact of screw configuration on the particle size distribution of granules produced by twin screw granulation. Int J Pharm. 2015;479:171–80.
Forster SP, Dippold E, Chiang T. Twin-screw melt granulation for oral solid pharmaceutical products. Pharmaceutics. 2021;13:665.
Portier C, Vervaet C, Vanhoorne V. Continuous twin screw granulation: a review of recent Progress and opportunities in formulation and equipment design. Pharmaceutics. 2021;13:668.
Zhao Y, Xie X, Zhao Y, Gao Y, Cai C, Zhang Q, et al. Effect of plasticizers on manufacturing ritonavir/copovidone solid dispersions via hot-melt extrusion: Preformulation, physicochemical characterization, and pharmacokinetics in rats. Eur J Pharm Sci. 2019;127:60–70.
Ghosh I, Vippagunta R, Li S, Vippagunta S. Key considerations for optimization of formulation and melt-extrusion process parameters for developing thermosensitive compound. Pharm Dev Technol. 2012;17:502–10.
Van Melkebeke B, Vermeulen B, Vervaet C, Remon JP. Melt granulation using a twin-screw extruder: A case study. Int J Pharm. 2006;326:89–93.
Srinivasan P, Almutairi M, Youssef AAA, Almotairy A, Bandari S, Repka MA. Numerical simulation of five different screw configurations used during the preparation of hot-melt extruded Kollidon® and Soluplus® based amorphous solid dispersions containing indomethacin. Journal of Drug Delivery Science and Technology. 2023;85: 104561.
Kumar A, Vercruysse J, Toiviainen M, Panouillot P-E, Juuti M, Vanhoorne V, et al. Mixing and transport during pharmaceutical twin-screw wet granulation: experimental analysis via chemical imaging. Eur J Pharm Biopharm. 2014;87:279–89.
Nandi U, Trivedi V, Ross SA, Douroumis D. Advances in twin-screw granulation processing Pharmaceutics. 2021;13:624.
Kallakunta VR, Tiwari R, Sarabu S, Bandari S, Repka MA. Effect of formulation and process variables on lipid based sustained release tablets via continuous twin screw granulation: a comparative study. Eur J Pharm Sci. 2018;121:126–38.
Seem TC, Rowson NA, Ingram A, Huang Z, Yu S, de Matas M, et al. Twin screw granulation—a literature review. Powder Technol. 2015;276:89–102.
Jakob B, Geilen T. Relevant process parameters for twin-screw compounding. 2018. https://www.thermofisher.com/document-connect/document-connect.html?url=https%3A%2F%2Fassets.thermofisher.com%2FTFS-Assets%2FCAD%2FApplication-Notes%2FLR70-relevant-process-parameters-twin-screw-compounding.pdf&title=UmVsZXZhbnQgUHJvY2VzcyBQYXJhbWV0ZXJzIGZvciBUd2luIFNjcmV3IENvbXBvdW5kaW5n.
Wang H, Vemula SK, Bandari S, Repka MA. Preparation of core-shell controlled release tablets using direct powder extrusion 3D printing techniques. Journal of Drug Delivery Science and Technology. 2023;88: 104896.
Okafor-Muo OL, Hassanin H, Kayyali R, ElShaer A. 3D printing of solid oral dosage forms: numerous challenges with unique opportunities. J Pharm Sci. 2020;109:3535–50.
Wang J, Zhang Y, Aghda NH, Pillai AR, Thakkar R, Nokhodchi A, et al. Emerging 3D printing technologies for drug delivery devices: current status and future perspective. Adv Drug Deliv Rev. 2021;174:294–316.
Solanki NG, Gumaste SG, Shah AV, Serajuddin ATM. Effects of surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate solid dispersion prepared by hot melt extrusion. II: rheological analysis and extrudability testing. Journal of Pharmaceutical Sciences. 2019;108:3063–73.
Alhijjaj M, Nasereddin J, Belton P, Qi S. Impact of processing parameters on the quality of pharmaceutical solid dosage forms produced by fused deposition modeling (FDM). Pharmaceutics. 2019;11:633.
Bandari S, Nyavanandi D, Dumpa N, Repka MA. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Adv Drug Deliv Rev. 2021;172:52–63.
Lakkala P, Munnangi SR, Bandari S, Repka M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. International Journal of Pharmaceutics: X. 2023;5: 100159.
Ding B, Yu Y, Geng S, Liu B, Hao Y, Liang G. Computational methods for the interaction between Cyclodextrins and natural compounds: technology, benefits, limitations, and trends. J Agric Food Chem. 2022;70:2466–82.
Manne ASN, Hegde AR, Raut SY, Rao RR, Kulkarni VI, Mutalik S. Hot liquid extrusion assisted drug-cyclodextrin complexation: a novel continuous manufacturing method for solubility and bioavailability enhancement of drugs. Drug Deliv and Transl Res. 2021;11:1273–87.
Donthi MR, Munnangi SR, Krishna KV, Marathe SA, Saha RN, Singhvi G, et al. Formulating ternary inclusion complex of sorafenib Tosylate using β-Cyclodextrin and hydrophilic polymers: physicochemical characterization and in vitro assessment. AAPS PharmSciTech. 2022;23:254.
Munnangi SR, Youssef AAA, Narala N, Lakkala P, Vemula SK, Alluri R, et al. Continuous manufacturing of solvent-free cyclodextrin inclusion complexes for enhanced drug solubility via hot-melt extrusion: a quality by design approach. Pharmaceutics. 2023;15:2203.
Thiry J, Krier F, Ratwatte S, Thomassin J-M, Jerome C, Evrard B. Hot-melt extrusion as a continuous manufacturing process to form ternary cyclodextrin inclusion complexes. Eur J Pharm Sci. 2017;96:590–7.
Malaquias LFB, Sá-Barreto LCL, Freire DO, Silva ICR, Karan K, Durig T, et al. Taste masking and rheology improvement of drug complexed with beta-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohyd Polym. 2018;185:19–26.
Granados PA, Pinho LAG, Sa-Barreto LL, Gratieri T, Gelfuso GM, Cunha-Filho M. Application of hot-melt extrusion in the complexation of naringenin with cyclodextrin using hydrophilic polymers. Adv Powder Technol. 2022;33: 103380.
Zhang T-Y, Du R-F, Wang Y-J, Hu J-L, Wu F, Feng Y. Research progress of preparation technology of ion-exchange resin complexes. AAPS PharmSciTech. 2022;23:105.
Munnangi SR, Youssef AAA, Narala N, Lakkala P, Narala S, Vemula SK, et al. Drug complexes: perspective from Academic Research and Pharmaceutical Market. Pharm Res. 2023;40:1519–40.
Tan DCT, Ong JJ, Gokhale R, Heng PWS. Hot melt extrusion of ion-exchange resin for taste masking. Int J Pharm. 2018;547:385–94.
Ghebre-Sellassie I, Terefe H, inventors; ExxPharma Therapeutics LLC, assignee. Tamper-resistant pharmaceutical dosage forms. United States patent US 9,770,514. 2017.
Wang X, Zhang L, Ma D, Tang X, Zhang Y, Yin T, et al. Characterizing and exploring the differences in dissolution and stability between crystalline solid dispersion and amorphous solid dispersion. AAPS PharmSciTech. 2020;21:262.
Agrawal AM, Dudhedia MS, Zimny E. Hot melt extrusion: development of an amorphous solid dispersion for an insoluble drug from mini-scale to clinical scale. AAPS PharmSciTech. 2016;17:133–47.
Darji M, Pradhan A, Vemula SK, Kolter K, Langley N, Repka MA. Development of delayed-release pellets of Ibuprofen using Kollicoat® MAE 100P via hot-melt extrusion technology. J Pharm Innov. 2023;18:1827–37.
Agrawal A, Dudhedia M, Deng W, Shepard K, Zhong L, Povilaitis E, et al. Development of tablet formulation of amorphous solid dispersions prepared by hot melt extrusion using quality by design approach. AAPS PharmSciTech. 2016;17:214–32.
Iyer R, Petrovska Jovanovska V, Berginc K, Jaklič M, Fabiani F, Harlacher C, et al. Amorphous solid dispersions (ASDs): the influence of material properties, manufacturing processes and analytical technologies in drug product development. Pharmaceutics. 2021;13:1682.
Auch C, Harms M, Mäder K. How changes in molecular weight and PDI of a polymer in amorphous solid dispersions impact dissolution performance. Int J Pharm. 2019;556:372–82.
Mohapatra S, Samanta S, Kothari K, Mistry P, Suryanarayanan R. Effect of polymer molecular weight on the crystallization behavior of indomethacin amorphous solid dispersions. Cryst Growth Des. 2017;17:3142–50.
Kyeremateng SO, Voges K, Dohrn S, Sobich E, Lander U, Weber S, et al. A hot-melt extrusion risk assessment classification system for amorphous solid dispersion formulation development. Pharmaceutics. 2022;14:1044.
Prasad E, Robertson J, Halbert GW. Mefenamic acid solid dispersions: impact of formulation composition on processing parameters, product properties and performance. Int J Pharm. 2022;616: 121505.
Pawar JN, Fule RA, Maniruzzaman M, Amin PD. Solid crystal suspension of Efavirenz using hot melt extrusion: exploring the role of crystalline polyols in improving solubility and dissolution rate. Mater Sci Eng, C. 2017;78:1023–34.
Reitz E, Vervaet C, Neubert RHH, Thommes M. Solid crystal suspensions containing griseofulvin – Preparation and bioavailability testing. Eur J Pharm Biopharm. 2013;83:193–202.
Zaworotko MJ. Molecules to crystals, crystals to molecules … and back again? Cryst Growth Des. 2007;7:4–9.
Yarava JR, Potnuru LR, Pahari B, Tothadi S, Ramanathan KV. Supramolecular synthon identification in azelaic acid—isonicotinamide. Journal of Magnetic Resonance Open. 2022;10–11: 100056.
Guo M, Sun X, Chen J, Cai T. Pharmaceutical cocrystals: a review of preparations, physicochemical properties and applications. Acta Pharmaceutica Sinica B. 2021;11:2537–64.
Panzade PS, Shendarkar GR, Kulkarni DA. Hot melt extrusion: an emerging green technique for the synthesis of high-quality pharmaceutical cocrystals. J Pharm Innov. 2022;17:283–93.
Narala S, Nyavanandi D, Srinivasan P, Mandati P, Bandari S, Repka MA. Pharmaceutical co-crystals, salts, and co-amorphous systems: a novel opportunity of hot-melt extrusion. Journal of Drug Delivery Science and Technology. 2021;61: 102209.
Narala S, Nyavanandi D, Alzahrani A, Bandari S, Zhang F, Repka MA. Creation of hydrochlorothiazide pharmaceutical cocrystals via hot-melt extrusion for enhanced solubility and permeability. AAPS PharmSciTech. 2022;23:1–11.
Thakkar R, Thakkar R, Pillai A, Ashour EA, Repka MA. Systematic screening of pharmaceutical polymers for hot melt extrusion processing: a comprehensive review. Int J Pharm. 2020;576: 118989.
Mandati P, Nyavanandi D, Narala S, Alzahrani A, Vemula SK, Repka MA. A comparative assessment of cocrystal and amorphous solid dispersion printlets developed by hot melt extrusion paired fused deposition modeling for dissolution enhancement and stability of Ibuprofen. AAPS PharmSciTech. 2023;24:203.
Chabalenge B, Korde S, Kelly AL, Neagu D, Paradkar A. Understanding matrix-assisted continuous co-crystallization using a data mining approach in quality by design (QbD). Cryst Growth Des. 2020;20:4540–9.
Shaikh R, Walker GM, Croker DM. Continuous, simultaneous cocrystallization and formulation of Theophylline and 4-Aminobenzoic acid pharmaceutical cocrystals using twin screw melt granulation. Eur J Pharm Sci. 2019;137: 104981.
Soliman II, Kandil SM, Abdou EM. Gabapentin-saccharin co-crystals with enhanced physicochemical properties and in vivo absorption formulated as oro-dispersible tablets. Pharm Dev Technol. 2020;25:227–36.
Gajda M, Nartowski KP, Pluta J, Karolewicz B. Continuous, one-step synthesis of pharmaceutical cocrystals via hot melt extrusion from neat to matrix-assisted processing—State of the art. Int J Pharm. 2019;558:426–40.
Maniruzzaman M, Nokhodchi A. Continuous manufacturing via hot-melt extrusion and scale up: regulatory matters. Drug Discovery Today. 2017;22:340–51.
Zecevic DE, Evans RC, Paulsen K, Wagner KG. From benchtop to pilot scale–experimental study and computational assessment of a hot-melt extrusion scale-up of a solid dispersion of dipyridamole and copovidone. Int J Pharm. 2018;537:132–9.
Matić J, Witschnigg A, Zagler M, Eder S, Khinast J. A novel in silico scale-up approach for hot melt extrusion processes. Chem Eng Sci. 2019;204:257–69.
Wesholowski J, Hoppe K, Nickel K, Muehlenfeld C, Thommes M. Scale-up of pharmaceutical hot-melt-extrusion: process optimization and transfer. Eur J Pharm Biopharm. 2019;142:396–404.
Roggo Y, Pauli V, Jelsch M, Pellegatti L, Elbaz F, Ensslin S, et al. Continuous manufacturing process monitoring of pharmaceutical solid dosage form: A case study. J Pharm Biomed Anal. 2020;179: 112971.
Khinast J, Bresciani M. Continuous manufacturing: definitions and engineering principles. In: Kleinebudde P, Khinast J, Rantanen J, Eds. John Wiley & Sons, Ltd; 2017. p. 1–31.
Panikar S, Li J, Rane V, Gillam S, Callegari G, Kurtyka B, et al. Integrating sensors for monitoring blend content in a pharmaceutical continuous manufacturing plant. Int J Pharm. 2021;606: 120085.
Liu P, Jin H, Chen Y, Wang D, Yan H, Wu M, et al. Process analytical technologies and self-optimization algorithms in automated pharmaceutical continuous manufacturing. Chin Chem Lett. 2024;35:108877.
Kurouski D, Dazzi A, Zenobi R, Centrone A. Infrared and Raman chemical imaging and spectroscopy at the nanoscale. Chem Soc Rev. 2020;49:3315–47.
Laske S, Paudel A, Scheibelhofer O, Sacher S, Hörmann T, Khinast J. A review of PAT strategies in secondary solid oral dosage manufacturing of small molecules. J Pharm Sci. 2017;106:667–712.
Wahlich J. Review: continuous manufacturing of small molecule solid oral dosage forms. Pharmaceutics. 2021;13:1311.
Su Q, Ganesh S, Moreno M, Bommireddy Y, Gonzalez M, Reklaitis GV, et al. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput Chem Eng. 2019;125:216–31.
Rossi CV. A comparative investment analysis of batch versus continuous pharmaceutical manufacturing technologies. J Pharm Innov. 2022;17:1373–91.
Emerging Technology Program. U.S. Food and Drug Administration. 2023. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/emerging-technology-program.
Fisher AC, Liu W, Schick A, Ramanadham M, Chatterjee S, Brykman R, et al. An audit of pharmaceutical continuous manufacturing regulatory submissions and outcomes in the US. Int J Pharm. 2022;622: 121778.
Guideline IC. Q13 on Continuous Manufacturing of Drug Substances and Drug Products. European Medicine Agency. 2023. https://www.ema.europa.eu/en/ich-guideline-q13-continuous-manufacturing-drug-substances-and-drug-products-scientific-guideline.
Ikeda S, Kobayashi M, Aoki S, Terukina T, Kanazawa T, Kojima H, et al. 3D-printed fast-dissolving oral dosage forms via fused deposition modeling based on sugar alcohol and poly(vinyl alcohol)—preparation, drug release studies and in vivo oral absorption. Pharmaceutics. 2023;15:395.
Tidau M, Finke JH. Modified release kinetics in dual filament 3D printed individualized oral dosage forms. Eur J Pharm Sci. 2022;175: 106221.
Yang Y, Wu H, Fu Q, Xie X, Song Y, Xu M, et al. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater Des. 2022;214: 110394.
Tiboni M, Campana R, Frangipani E, Casettari L. 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. Int J Pharm. 2021;596: 120290.
Alzahrani A, Youssef AAA, Nyavanandi D, Tripathi S, Bandari S, Majumdar S, et al. Design and optimization of ciprofloxacin hydrochloride biodegradable 3D printed ocular inserts: Full factorial design and in-vitro and ex-vivo evaluations: Part II. Int J Pharm. 2023;631: 122533.
Elkanayati RM, Chambliss WG, Omari S, Almutairi M, Repka MA, Ashour EA. Mucoadhesive buccal films for treatment of xerostomia prepared by coupling HME and 3D printing technologies. Journal of Drug Delivery Science and Technology. 2022;75: 103660.
Author information
Authors and Affiliations
Contributions
Conceptualization: Hemlata Patil, Sateesh Kumar Vemula, and Michael A. Repka. Writing—original draft: Sagar Narala, Preethi Lakkala, Siva Ram Munnangi, Nagarjuna Narala, Miguel O Jara, Hemlata Patil, and Sateesh Kumar Vemula. Writing—review and editing: Hemlata Patil, Sagar Narala, Sateesh Kumar Vemula, and Michael A. Repka. Supervision: Robert O. Williams III, Hibreniguss Terefe, and Michael A. Repka.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patil, H., Vemula, S.K., Narala, S. et al. Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation—Where Are We Now?. AAPS PharmSciTech 25, 37 (2024). https://doi.org/10.1208/s12249-024-02749-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-024-02749-2