Abstract
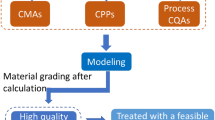
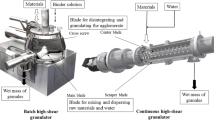
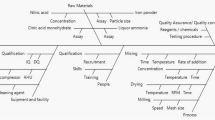
Traditional methods of producing Xiaochaihu (XCH) capsules, a traditional Chinese medicine, are time-consuming, costly, and labor-intensive, which is not conductive to modernizing TCM. To address the challenges, new fluid-bed granulation and drying processes with water as the binder were developed and optimized guided by the principles of Quality by Design (QbD) in this study. Ishikawa diagram was applied to conduct a preliminary risk assessment, followed by 6-factor definitive screening design (DSD) serving as a QbD statistical tool to develop and optimize the new processes. Multiple potential factors and interactions were studied with a small number of experiments using the DSD. This study identified critical process parameters (CPPs), established quadratic regression models to reveal CPP-critical quality attributes (CQAs) connections within the DSD framework, and defined a dependable design space. Processes conducted by parameter combinations in the design space produced qualified granules with production yield and raw material utilization higher than 90% and moisture content lower than 4%. Furthermore, quantitative analysis of baicalin of all the granules ensured qualified contents of active pharmaceutical ingredient. The newly developed processes for XCH capsules, with advantages of shorter time, environmental friendliness, and decreased cost, exemplify the effective application of QbD and design of experiments (DoE) methodologies in the modernization of TCM manufacturing processes.
Graphical Abstract









Similar content being viewed by others
References
The Chinese Pharmacopoeia. Commission CP, editor. Beijing, China: China Medical Science Press; 2020. 604 p.
Zheng C. The effects of the Xiaochaihu capsule on immunoglobulin A nephropathy. Clin J Chin Med. 2020;12(26):63–4.
Wu L, Geng B, Zhang R, Yong D, Xu J. Study on antibacterial and liver protective effects of Xiaochaihu capsule. Pharmacol Clin Chin Materia Med. 1999;06:3–5.
Zhang Y, Zhou X, Shao Q, Ze F, Wang S. Anti-inflammatory effect of Xiaochaihutang in rats with collagen-induced arthritis and the mechanism. Immunol J. 2015;31(09):781–5.
Liu J, Sun R. Protective effect of Xiaochaihu decoction on non-alcoholic steatohepatitis model mice. Chin Tradit Herb Drugs. 2020;51(14):3708–16.
Li L, Zhang Y, Wang X. Anti-tumor effect of Bupleuri Radix, Scutellariae Radix and Xiaochaihu capsule. Tradit Chin Med Res. 2013;26(08):79–80.
Su Z. Investigation and influencing factors analysis of Chinese medicine decoction taking compliance. J Tradit Chin Med Manag. 2022;30(13):35–7.
Fries L, Antonyuk S, Heinrich S, Dopfer D, Palzer S. Collision dynamics in fluidised bed granulators: a DEM-CFD study. Chem Eng Sci. 2013;86:108–23.
Morin G, Briens L. A comparison of granules produced by high-shear and fluidized-bed granulation methods. AAPS PharmSciTech. 2014;15(4):1039–48.
Kwon HJ, Heo E-J, Kim Y-H, Kim S, Hwang Y-H, Byun J-M, et al. Development and evaluation of poorly water-soluble celecoxib as solid dispersions containing nonionic surfactants using fluidized-bed granulation. Pharmaceutics. 2019;11(3):136.
Goracinova K, Klisarova L, Simov A, Fredro-Kumbaradzi E, Petrusevska-Tozi L. Characterization of fluid bed prepared granulates with verapamil hydrochloride as active substance. Acta Pharmaceutica (Zagreb). 1996;46(2):147–53.
Consiglieri V, Rivas P, Lopez P, Sampaio M, Spricigo R, Mourao S, et al. Developments of fluconazole granulation by fluid bed for capsules and tablets manufacturing. Latin Am J Pharm. 2007;26(1):20–5.
Thapa P, Tripathi J, Jeong SH. Recent trends and future perspective of pharmaceutical wet granulation for better process understanding and product development. Powder Technol. 2019;344:864–82.
Tamrakar A, Ramachandran R. CFD–DEM–PBM coupled model development and validation of a 3D top-spray fluidized bed wet granulation process. Comput Chem Eng. 2019;125:249–70.
Menon A, Dhodi N, Mandella W, Chakrabarti S. Identifying fluid-bed parameters affecting product variability. Int J Pharm. 1996;140(2):207–18.
Liu H, Wang K, Schlindwein W, Li M. Using the Box–Behnken experimental design to optimise operating parameters in pulsed spray fluidised bed granulation. Int J Pharm. 2013;448(2):329–38.
Lipsanen T, Antikainen O, Räikkönen H, Airaksinen S, Yliruusi J. Novel description of a design space for fluidised bed granulation. Int J Pharm. 2007;345(1):101–7.
Debevec V, Srcic S, Horvat M. Scientific, statistical, practical, and regulatory considerations in design space development. Drug Dev Ind Pharm. 2018;44(3):349–64.
Dhoot AS, Fernandes GJ, Naha A, Rathnanand M, Kumar L. Design of experiments in pharmaceutical development. Pharm Chem J. 2019;53(8):730–5.
ICH Q8 (R2) 2009. Q8 guideline: pharmaceutical development. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073507.pdf. Accessed Nov 2009, ICH, Rev 2.
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91.
Jelena D, Djordje M, Marko K, Ivana V, Ivana M, Svetlana I. Design space approach in optimization of fluid bed granulation and tablets compression process. Sci World J. 2012;2012:185085.
Närvänen T, Antikainen O, Yliruusi J. Predicting particle size during fluid bed granulation using process measurement data. AAPS PharmSciTech. 2009;10(4):1268–75.
Lourenço V, Lochmann D, Reich G, Menezes JC, Herdling T, Schewitz J. A quality by design study applied to an industrial pharmaceutical fluid bed granulation. Eur J Pharm Biopharm. 2012;81(2):438–47.
Rambali B, Baert L, Massart DL. Using experimental design to optimize the process parameters in fluidized bed granulation on a semi-full scale. Int J Pharm. 2001;220(1):149–60.
Dacanal GC, Menegalli FC. Selection of operational parameters for the production of instant soy protein isolate by pulsed fluid bed agglomeration. Powder Technol. 2010;203(3):565–73.
Zhao J, Li W, Qu H, Tian G, Wei Y. Application of definitive screening design to quantify the effects of process parameters on key granule characteristics and optimize operating parameters in pulsed-spray fluid-bed granulation. Particuology. 2018;43:56–65.
Rambali B, Baert L, Thoné D, Massart DL. Using experimental design to optimize the process parameters in fluidized bed granulation. Drug Dev Ind Pharm. 2001;27(1):47–55.
Jiang S, Yang L, Wei X. Preparation process study of ibuprofen arginine granules based on quality by design approach. Chin J Pharm. 2022;53(10):1446–52.
Meshali M, El-Banna HM, El-Sabbagh H. Use of a fractional factorial design to evaluate granulations prepared in a fluidized bed. Die Pharmazie. 1983;38(5):323–5.
Lipps DM, Sakr AM. Characterization of Wet Granulation process parameters using response surface methodology. 1 Top-spray fluidized bed. J Pharm Sci. 1994;83(7):937–47.
Jones B, Nachtsheim CJ. A class of three-level designs for definitive screening in the presence of second-order effects. J Qual Technol. 2011;43(1):1–15.
Erler A, de Mas N, Ramsey P, Henderson G. Efficient biological process characterization by definitive-screening designs: the formaldehyde treatment of a therapeutic protein as a case study. Biotechnol Lett. 2013;35(3):323–9.
Takagaki K, Ito T, Arai H, Obata Y, Takayama K, Onuki Y. The usefulness of definitive screening design for a quality by design approach as demonstrated by a pharmaceutical study of orally disintegrating tablet. Chem Pharm Bull. 2019;67(10):1144–51.
Tian G, Wei Y, Zhao J, Li W, Qu H. Application of near-infrared spectroscopy combined with design of experiments for process development of the pulsed spray fluid bed granulation process. Powder Technol. 2018;339:521–33.
Benjasirimongkol P, Piriyaprasarth S, Moribe K, Sriamornsak P. Use of Risk Assessment and Plackett-Burman design for developing resveratrol spray-dried emulsions: a quality-by-design approach. AAPS PharmSciTech. 2018;20(1):14.
Gao JZH, Jain A, Motheram R, Gray DB, Hussain MA. Fluid bed granulation of a poorly water soluble, low density, micronized drug: comparison with high shear granulation. Int J Pharm. 2002;237(1):1–14.
Shao J, Qu H, Gong X. Comparison of two algorithms for development of design space-overlapping method and probability-based method. China J Chin Materia Med. 2018;43(10):2074–80.
Chordiya M, Gangurde H, Sancheti V. Quality by design: a roadmap for quality pharmaceutical products. J Rep Pharma Sci. 2019;8(2):289.
Fukuda IM, Pinto CFF, Moreira CdS, Saviano AM, Lourenço FR. Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci. 2018;54:e01006.
Kim JY, Choi DH. Quality by design approach with multivariate analysis and artificial neural network models to understand and control excipient variability. J Pharm Investig. 2023;53(3):389–406.
Parikh DM. Handbook of pharmaceutical granulation technology. 3rd ed. Ellicott City, Maryland, USA: CRC Press; 2010.
Zeng J, Ming L, Wang J, Huang T, Liu B, Feng L, et al. Empirical prediction model based process optimization for droplet size and spraying angle during pharmaceutical fluidized bed granulation. Pharm Dev Technol. 2020;25(6):720–8.
Teresa J, Christelle T, Elisabeth D. Particles agglomeration in a conical fluidized bed in relation with air temperature profiles. Chem Eng Sci. 2006;61(18):5954–61.
Bemrose CR, Bridgwater J. A review of attrition and attrition test methods. Powder Technol. 1987;49(2):97–126.
Stefan H, Jan B, Markus H, Matthias I, Mirko P, Lothar M. Study of dynamic multi-dimensional temperature and concentration distributions in liquid-sprayed fluidized beds. Chem Eng Sci. 2003;58(23):5135–60.
Chen W, Chang S-Y, Kiang S, Early W, Paruchuri S, Desai D. The measurement of spray quality for pan coating processes. J Pharm Innov. 2008;3(1):3–14.
Henrik E, Jussi L, Osmo A, Heikki R, Jyrki H, Jouko Y. In situ droplet size and speed determination in a fluid-bed granulator. Int J Pharm. 2010;391(1):148–54.
Karine L, Cédric B, Thierry G, Jérôme B, Pierre G, Maurice B. Experimental measurement of droplet vaporization kinetics in a fluidized bed. Chem Eng Process: Process Intensif. 2004;43(6):693–9.
Qiu F, Tang X, He Z, Li H. Stability of baicalin aqueous solution by validated RP-HPLC. J Chin Pharm Sci. 2004;02:134–7.
Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, et al. Understanding Pharmaceutical Quality by Design. AAPS J. 2014;16(4):771–83.
Sonawane A, Pathak S, Pradhan RC. Bioactive compounds in bael fruit pulp waste: ultrasound-assisted extraction, characterization, modeling, and optimization approaches. Biointerface Res Appl Chem. 2021;11(2):9318–34.
Funding
This work was financially supported by the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine [ZYYCXTD-D-202002].
Author information
Authors and Affiliations
Contributions
KT: methodology, validation, investigation, and writing—original draft; HF: acquisition of data for the work; GW: project administration; PG: methodology; YX: methodology; PZ: resources; XG: methodology, data curation; HQ: the conception or design of the work, writing—reviewing and editing—supervision, and funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teng, K., Fu, H., Wu, G. et al. QbD-Guided Traditional Chinese Medicine Manufacturing Process: Development and Optimization of Fluid-Bed Granulation and Drying Processes for Xiaochaihu Capsules. AAPS PharmSciTech 24, 210 (2023). https://doi.org/10.1208/s12249-023-02663-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02663-z