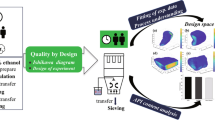
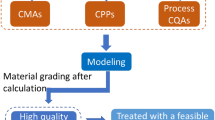
Abstract
It is challenging for a small-scale pharmaceutical manufacturer to comply with evolving regulatory standards. Quality by design (QbD) is a simple solution. The purpose of this study is to demonstrate the use of QbD in optimizing the synthesis of Ferric Ammonium Citrate which will help manufacturers ensure higher regulatory compliance. Three key starting materials (KSM) were reviewed for their synthetic pathways. QbD approach was used to develop the process. The central composite design was used to design experiments of two variables at three levels. Percentage yield, description, and iron content were recorded. Optimized values of variable factors were obtained by contour plots, 3D response surface graphs, and prediction profiler. Repeatability and scale-up were performed with the optimized values. The process was developed using iron powder as KSM and a combination of 50% v/v nitric acid and air bubbling as an oxidizing agent. Risk assessment tools led to selection of variable factors like the quantity of nitric acid and time for air bubbling for optimization. Percentage yield increased with an increase in air bubbling time, whereas the description did not comply with an increase in nitric acid content. All the experiments resulted in iron content within the range. Contour plots and prediction profiler confirmed the optimized values of content of nitric acid as 125 g and air bubbling time of 19 h for a batch size of 400 g. An optimized process leads to fewer variations and better quality assurance. This leads to ensuring higher regulatory compliance.



Similar content being viewed by others
References
Abraham J (2009) International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. In: Handbook of transnational economic governance regimes. pp 1041–1053
am Ende D, Bronk KS, Mustakis J, O’Connor G, Santa Maria CL, Nosal R, Watson TJN (2007) API quality by design example from the torcetrapib manufacturing process. J Pharm Innov 2(3–4):71–86. https://doi.org/10.1007/s12247-007-9015-x
Amasya G, Aksu B, Badilli U, Onay-Besikci A, Tarimci N (2019) QbD guided early pharmaceutical development study: production of lipid nanoparticles by high pressure homogenization for skin cancer treatment. Int J Pharm 563:110–121. https://doi.org/10.1016/j.ijpharm.2019.03.056
Anurag S, Yogesh A, Sunilkumar G, Rajinder S, Nandu B, Girji S (2017) Process for the formation of pharmaceutical grade ferric citrate. WO patent 2017/021921 Al, 9 Feb 2017
Baber N (1994) International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Br J Clin Pharmacol 37(5):401–404. https://doi.org/10.1111/j.1365-2125.1994.tb05705.x
Chen Z, Wang H, Wu S, Wang J, Zhang C, Yang H, Wang Z (2020) Optimization and process improvement for LCZ696 by employing quality by design (QbD) principles. Tetrahedron 76(46):131558. https://doi.org/10.1016/j.tet.2020.131558
Cimarosti Z, Bravo F, Castoldi D, Tinazzi F, Provera S, Perboni A, Papini D, Westerduin P (2010) Application of the QbD principles in the development of the casopitant mesylate manufacturing process. Process research studies for the definition of the control strategy of some drug substance-CQAs for stages 2a, 2b, and 2c. Org Process Res Dev 14(4):805–814. https://doi.org/10.1021/op1000622
Colombo S, Beck-Broichsitter M, Bøtker JP, Malmsten M, Rantanen J, Bohr A (2018) Transforming nanomedicine manufacturing toward quality by design and microfluidics. Adv Drug Deliv Rev 128:115–131. https://doi.org/10.1016/j.addr.2018.04.004
Connelly NG, Geiger WE (1996) Chemical redox agents for organometallic chemistry. Chem Rev 96(2):877–910. https://doi.org/10.1021/cr940053x
Debevec V, Srčič S, Horvat M (2018) Scientific, statistical, practical, and regulatory considerations in design space development. Drug Dev Ind Pharm 44:349–364
Diab S, McQuade DT, Gupton BF, Gerogiorgis DI (2019) Process design and optimization for the continuous manufacturing of nevirapine, an active pharmaceutical ingredient for HIV treatment. Org Process Res Dev 23(3):320–333. https://doi.org/10.1021/acs.oprd.8b00381
Dumarey M, Hermanto M, Airiau C, Shapland P, Robinson H, Hamilton P, Berry M (2019) Advances in continuous active pharmaceutical ingredient (API) manufacturing: real-time monitoring using multivariate tools. J Pharm Innov 14(4):359–372. https://doi.org/10.1007/s12247-018-9348-7
FDA (2006) Guidance for industry—quality systems approach to pharmaceutical CGMP regulations. Risk Manag, p 32
FDA (2019) Quality considerations for continuous manufacturing guidance for industry. Food Drug Adm, pp 1–27
Grangeia HB, Silva C, Simões SP, Reis MS (2020) Quality by design in pharmaceutical manufacturing: a systematic review of current status, challenges and future perspectives. Eur J Pharm Biopharm 147:19–37. https://doi.org/10.1016/j.ejpb.2019.12.007
Hani U, Osmani RAM, Alqahtani A, Ghazwani M, Rahamathulla M, Almordy SA, Alsaleh HA (2020) 23 Full factorial design for formulation and evaluation of floating oral in situ gelling system of piroxicam. J Pharm Innov. https://doi.org/10.1007/s12247-020-09471-z
Hegde N, Juvale K, Prabhakar B (2021) Formulation and optimization of gefitinib-loaded nanosuspension prepared using a newly developed dendritic lipopeptide oligomer material. Chem Pap 75(5):2007–2022. https://doi.org/10.1007/S11696-020-01453-2
Hsiao Y, Chiu H, Wang H, Chang Y (2005) Pharmaceutical- grade ferric citrate. US Patent 6,903,235 B2, 7 June 2005
ICH (2012) Developemnt and manufacture of drug substances (chemical entities and biotechnological/biological entities. International conference on harmonization tripartite guideline
ICH (2019) Technical and regulatory considerations for pharmaceutical product lifecycle management. International conference on harmonization tripartite guideline.
Kapic S, Nekola I, Jovic F, Mihovilovic M (2018) Process optimization and DoE application in the synthesis of Rociletinib intermediate. Chem Biochem Eng Q 32(2):167–175
Kaur A, Bhoop BS, Chhibber S, Sharma G, Gondil VS, Katare OP (2017) Supramolecular nano-engineered lipidic carriers based on diflunisal-phospholipid complex for transdermal delivery: QbD based optimization, characterization and preclinical investigations for management of rheumatoid arthritis. Int J Pharm 533(1):206–224. https://doi.org/10.1016/j.ijpharm.2017.09.041
Kelley B, Cromwell M, Jerkins J (2016) Integration of QbD risk assessment tools and overall risk management. Biologicals 44(5):341–351. https://doi.org/10.1016/j.biologicals.2016.06.001
Leyva E, Moctezuma E, Baines KM, Noriega S, Zarazua E (2017) A review on chemical advanced oxidation processes for pharmaceuticals with paracetamol as a model compound. Reaction conditions, intermediates and total mechanism. Curr Org Chem 22(1):2–17. https://doi.org/10.2174/1385272821666171019145520
Li S, Chen J, Feng C, Yang W, Ji M (2019) A new route for the synthesis of Palbociclib. Chem Pap 73(12):3043–3051. https://doi.org/10.1007/S11696-019-00841-7
Liu (2013) A kind of preparation method of soluble citrate (2013). CN patent 104418729A, 26 Aug 2013
Mallu UR (2015) API supplier change or addition of alterate API supplier in generic drug products: Cost, quality and regulatory factors. Pharm Anal Acta 06(05):364. https://doi.org/10.4172/2153-2435.1000364
Mamidi HK, Palekar S, Nukala PK, Mishra SM, Patki M, Fu Y, Supner P, Chauhan G, Patel K (2021) Process optimization of twin-screw melt granulation of fenofibrate using design of experiment (DoE). Int J Pharm 593:120101. https://doi.org/10.1016/j.ijpharm.2020.120101
Mei Z, Sun Y (2009) The production method for ferric ammonium citrate. CN Patent 101704738B, 26 Nov 2009
Metil DS, Sonawane SP, Pachore SS, Mohammad A, Dahanukar VH, McCormack PJ, Reddy CV, Bandichhor R (2018) Synthesis and optimization of canagliflozin by employing quality by design (QbD) principles. Org Process Res Dev 22(1):27–39. https://doi.org/10.1021/ACS.OPRD.7B00281
Mishra V, Thakur S, Patil A, Shukla A (2018) Quality by design (QbD) approaches in current pharmaceutical set-up. Expert Opin Drug Deliv 15:737–758
Mohammed AQ, Sunkari PK, Srinivas P, Roy AK (2015) Quality by design in action 1: controlling critical quality attributes of an active pharmaceutical ingredient
Monograph on iron and ammonium citrate, Indian Pharmacopoeia 2018, pp 2314–2315
Naviglio D, Salvatore MM, Limatola M, Langella C, Faralli S, Ciaravolo M, Andolfi A, Salvatore F, Gallo M (2018) Iron (II) citrate complex as a food supplement: synthesis, characterization and complex stability. Nutrients 10(11):1–11. https://doi.org/10.3390/nu10111647
Phansalkar MS, Shetgiri NP (2005) Process considerations during API development. Pharm Technol Eur 17(2):19–27
Politis SN, Colombo P, Colombo G, Rekkas DM (2017) Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm 43:889–901
Popkin ME, Borman PJ, Omer BA, Seibert KD, Srivastava S, Lepore JV, Hobson L, Donaubauer J, Curran T, Ide N, Tymonko S, Looker A, Kallemeyn JM (2018) The delivery of flexibility from the application of QbD to API development. J Pharm Innov 13(4):367–372. https://doi.org/10.1007/s12247-018-9339-8
Portillo PM, Ierapetritou M, Tomassone S, Mc Dade C, Clancy D, Avontuur PPC, Muzzio FJ (2008) Quality by design methodology for development and scale-up of batch mixing processes. J Pharm Innov 3(4):258–270. https://doi.org/10.1007/s12247-008-9048-9
Saka OM, Öz UC, Küçüktürkmen B, Devrim B, Bozkır A (2020) Central composite design for optimization of Zoledronic acid loaded PLGA nanoparticles. J Pharm Innov 15(1):3–14. https://doi.org/10.1007/s12247-018-9365-6
Salazar J, Heinzerling O, Müller RH, Möschwitzer JP (2011) Process optimization of a novel production method for nanosuspensions using design of experiments (DoE). Int J Pharm 420(2):395–403. https://doi.org/10.1016/j.ijpharm.2011.09.003
Schaefer C, Clicq D, Lecomte C, Merschaert A, Norrant E, Fotiadu F (2014) A process analytical technology (PAT) approach to control a new API manufacturing process: development, validation and implementation. Talanta 120:114–125. https://doi.org/10.1016/j.talanta.2013.11.072
Somma R (2007) Development knowledge can increase manufacturing capability and facilitate quality by design. J Pharm Innov 2(3–4):87–92. https://doi.org/10.1007/s12247-007-9017-8
Tenci M, Rossi S, Bonferoni MC, Sandri G, Mentori I, Boselli C, Cornaglia AI, Daglia M, Marchese A, Caramella C, Ferrari F (2017) Application of DoE approach in the development of mini-capsules, based on biopolymers and manuka honey polar fraction, as powder formulation for the treatment of skin ulcers. Int J Pharm 516(1–2):266–277. https://doi.org/10.1016/j.ijpharm.2016.10.050
World Health Organization (2011) WHO expert committee on specifications for pharmaceutical preparations. World Health Organ Tech Rep Ser
Zhang Y, Song W, Ban Y (2017), A kind of ferric citrate preparation technology. CN patent 107417513A, 22 Aug 2017
Zhang D, Su J (2015) Investigation of reduction process and related impurities in ezetimibe. J Pharm Biomed Anal 107:355–363. https://doi.org/10.1016/j.jpba.2015.01.008
Zhao S, Wen X, Chang S (2018), The synthetic method of ferric citrate. CN patent 108456137A, 3 May 2018
Acknowledgements
The authors would like to thank Dr. Kapil Juvale, Assistant Professor, Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management for his suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jalundhwala, F., Londhe, V. & Shah, B. Ensuring regulatory compliance by quality by design (QbD) approach to optimize the manufacturing process of API: ferric ammonium citrate as an example. Chem. Pap. 77, 1469–1477 (2023). https://doi.org/10.1007/s11696-022-02569-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02569-3