Abstract
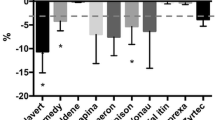
It is well known that the splitting of tablets can bring serious risks to the health of the treated animals, e.g., the possible adverse reactions caused by overdoses of fenbendazole or aspirin. In this regard, this work aimed to evaluate, for the first time, the splitting behavior of commercial veterinary tablets and identifying the technological aspects that interfere in this process. Tablets were cut in halves using a tablet splitter and were analyzed regarding mass variation, mass loss, friability, and hardness. Microstructural and morphological evaluations were also performed. For most of the tablets, organic flavor additives provided more uniformity and cohesive matrix, which preserved its hardness after the cut and led to subdivision results within acceptable limits for mass measurements and friability. Apart from the microstructure, the most critical technological aspect for a correct splitting performance in such tablets was the presence of a score. Thus, the results presented here allow us to guide the manufacturing of veterinary drug products in order to produce tablets more adapted to the splitting process.





Similar content being viewed by others
References
O’Haire M. Companion animals and human health: Benefits, challenges, and the road ahead. J Vet Behav. 2010;5(5):226–34. https://doi.org/10.1016/j.jveb.2010.02.002.
Bimbatti Mattos LF. Private mergers and acquisitions in the United States: A high-level overview of veterinary health industry deals and its recent consolidation wave. J Anim Environ Law. 2019;11(1):28–51.
Young NW, Royal KD, Davidson GS. Baseline knowledge of potential pet toxins: a survey of pharmacists. Pharm Pract (Granada). 2017;15(4):1058. https://doi.org/10.18549/PharmPract.2017.04.1058.
Siven M, Savolainen S, Rantila S, Mannikko S, Vainionpaa M, Airaksinen S, et al. Difficulties in administration of oral medication formulation to pet cats: an e-survey of cat owners. Vet Rec. 2017;180(10):250. https://doi.org/10.1136/vr.103991.
Song Y, Peressin K, Wong PY, Page SW, Garg S. Key considerations in designing oral drug delivery systems for dogs. J Pharm Sci. 2016;105(5):1576–85. https://doi.org/10.1016/j.xphs.2016.03.007.
Savolainen S, Hautala J, Junnila J, Airaksinen S, Juppo AM, Raekallio M, et al. Acceptability of flavoured pharmaceutically non-active mini-tablets in pet cats tested with a rapid 3-portal acceptance test with and without food. Vet Anim Sci. 2019;7:100054. https://doi.org/10.1016/j.vas.2019.100054.
Davidson G. Introduction to Veterinary Pharmacy. In: Measley KL, editor. Pharmacotherapeutics for Veterinary Dispensing. 1st ed. New York: John Wiley & Sons Inc.; 2019. p. 1–24.
Ahmed I, Kasraian K. Pharmaceutical challenges in veterinary product development. Adv Drug Deliv Rev. 2002;54(6):871–82. https://doi.org/10.1016/S0169-409X(02)00074-1.
Fleischer S, Sharkey M, Mealey K, Ostrander EA, Martinez M. Pharmacogenetic and metabolic differences between dog breeds: their impact on canine medicine and the use of the dog as a preclinical animal model. AAPS J. 2008;10(1):110–9. https://doi.org/10.1208/s12248-008-9011-1.
Siroka Z, Svobodova Z. The toxicity and adverse effects of selected drugs in animals – overview. Pol J Vet Sci. 2013;16(1):181–91. https://doi.org/10.2478/pjvs-2013-002.
Poppenga RH. Herbal medicine: Potential for intoxication and interactions with conventional drugs. Clin Tech Small Anim Pract. 2002;17(1):6–18. https://doi.org/10.1053/svms.2002.27785.
Geary TG, Thompson DP. Development of antiparasitic drugs in the 21st century. Vet Parasitol. 2003;115(2):167–84. https://doi.org/10.1016/s0304-4017(03)00205-x.
Woods DJ, Vaillancourt VA, Wendt JA, Meeus PF. Discovery and development of veterinary antiparasitic drugs: past, present and future. Future Med Chem. 2011;3(7):887–96. https://doi.org/10.4155/fmc.11.39.
Elliott I, Mayxa M, Yeuichaixong S, Lee SJ, Newton PN. The practice and clinical implications of tablet splitting in international health. Tropical Med Int Health. 2014;19(7):754–60. https://doi.org/10.1111/tmi.12309.
Cunha-Filho M, Gelfuso GM, Gratieri T. Subdivision of modified-release tablets: state-of-the-art and future perspectives. Ther Deliv. 2020;11(5):285–7. https://doi.org/10.4155/tde-2020-0006.
Teixeira MT, Sa-Barreto LL, Taveira SF, Gratieri T, Gelfuso GM, Marreto RN, et al. The influence of matrix technology on the subdivision of sustained release matrix tablets. AAPS PharmSciTech. 2020;21(1):8. https://doi.org/10.1208/s12249-019-1554-1.
European Medicines Agency/ International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). Test procedures and acceptance criteria for new veterinary drug substances and new medicinal products: chemical substances. In VICH GL 39. European Medicines Agency 2006. https://www.ema.europa.eu/en/documents/scientific-guideline/vich-gl39-test-procedures-acceptance-criteria-new-veterinary-drug-substances-new-medicinal-products_en.pdf. Accessed 18 April 2020
FDA. Guidance for Industry. Tablet Scoring: Nomenclature, Labeling, and Data for Evaluation. U.S. Department of Health and Human Services Food and Drug Administration. 2013. https://www.fda.gov/media/81626/download. (Accessed 22 Oct, 2020).
Council of Europe. Monograph Tablets. European Pharmacopoeia. 10th ed. Strasbourg: Council of Europe; 2020. https://pheur.edqm.eu/subhome/10-0. (Accessed 22 Oct, 2020).
Abu-Geras D, Hadziomerovic D, Leau A, Nazim Khan R, Gudka S, Locher C, et al. Accuracy of tablet splitting and liquid measurements: an examination of why, what and how. J Pharm Pharmacol. 2017;69(5):603–12. https://doi.org/10.1111/jphp.1271.
Seong S, Shin JY, Kim D, Song I, Sun S, Kim I, et al. The effect of tablet splitting on the mass loss, uniformity, and stability: by hand or splitter? J Asian Assoc Sch Pharm. 2019;8:7–14.
Gharaibeh SF, Tahaineh L. Effect of different splitting techniques on the characteristics of divided tablets of five commonly split drug products in Jordan. Pharm Pract (Granada). 2020;18(2):1776. https://doi.org/10.18549/pharmpract.2020.2.1776.
United States Pharmacopeia, 2005. General Chapters 1216 Tablet friability. In United States Pharmacopeia https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/g06_pf_ira_32_2_2006.pdf. Accessed 28 April 2020
Cunha-Filho M, Teixeira MT, Santos-Rosales V, Sa-Barreto LL, Marreto RN, Martin-Pastor M, et al. The subdivision behavior of polymeric tablets. Int J Pharm. 2019;568:118554. https://doi.org/10.1016/j.ijpharm.2019.118554.
Sovány T, Kása J, Vakli K, Pintye-Hódi K. X-ray computed microtomography for determination of relationships between structure and breaking of scored tablets. X-Ray Spectrom. 2009;38(6):505–9. https://doi.org/10.1002/xrs.1205.
Zaid AN, Hawari R, Malkieh N, Natshih Y, Yousef A, Jaradat N, et al. Impact of formulation variables on weight uniformity of scored tablets using factorial design. Pak J Pharm Sci. 2019;32(5):2501–7.
Eserian JK, Lombardo M. Tablet subdivision: far beyond the splitting technique. Int J Pharm. 2014;476(1-2):77. https://doi.org/10.1016/j.ijpharm.2014.09.046.
Gupta A, Chidambaram N, Khan MA. An index for evaluating difficulty of chewing index for chewable tablets. Drug Dev Ind Pharm. 2015;41(2):239–43. https://doi.org/10.3109/03639045.2013.858736.
Teixeira MT, Sá-Barreto LC, Gratieri T, Gelfuso GM, Silva IC, Cunha-Filho MS. Key technical aspects influencing the accuracy of tablet subdivision. AAPS PharmSciTech. 2017;18(4):1393–401. https://doi.org/10.1208/s12249-016-0615-y.
Sovány T, Kása P Jr, Pintye-Hódi K. Comparison of the halving of tablets prepared with eccentric and rotary tablet presses. AAPS PharmSciTech. 2009;10(2):430–6. https://doi.org/10.1208/s12249-009-9225-2.
Pereira GRS, Taveira SF, Cunha-Filho M, Marreto RN. The effects of fillers and binders on the accuracy of tablet subdivision. AAPS PharmSciTech. 2018;19(7):2929–33. https://doi.org/10.1208/s12249-018-1144-7.
Teixeira MT, Sa-Barreto LL, Silva IC, Gratieri T, Gelfuso GM, Marreto RN, et al. The influence of porosity on tablet subdivision. Particuology. 2020;53:192–6. https://doi.org/10.1016/j.partic.2020.06.001.
Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Spec Pharm. 2009;15(3):253–61. https://doi.org/10.18553/jmcp.2009.15.3.253.
van der Steen KC, Frijlink HW, Schipper CMA, Barends DM. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech. 2010;11(1):126–32. https://doi.org/10.1208/s12249-009-9365-4.
Sovány T, Kása P, Pintye-Hódi K. Modeling of subdivision of scored tablets with the application of artificial neural networks. J Pharm Sci. 2010;99(2):905–15. https://doi.org/10.1002/jps.21853.
Tahaineh LM, Gharaibeh SF. Tablet splitting and weight uniformity of half-tablets of four medications in pharmacy practice. J Pharm Pract. 2012;25(4):471–6. https://doi.org/10.1177/0897190012442716.
Katakam LNR. Split-half tablets: a complete review for analytical testing. Asian J Pharm Clin Res. 2019;12(9):27–38. https://doi.org/10.22159/ajpcr.2019.v12i9.34601.
van Vooren L, De Spiegeleer B, Thonissen T, Joye P, van Durme J, Slegers G. Statistical analysis of tablet breakability methods. J Pharm Pharm Sci. 2002;5(2):190–8.
Andersson AC, Lindemalm S, Eksborg S. Dividing the tablets for children - Good or Bad? Pharm Methods. 2016;7(1):23–7. https://doi.org/10.5530/phm.2016.7.4.
McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18(1):193–7. https://doi.org/10.1002/j.1875-9114.1998.tb03838.x.
Temer AC, Teixeira MT, Sa-Barreto LL, Gratieri T, Gelfuso GM, Silva IC, et al. Subdivision of tablets containing modified delivery technology: the case of orally disintegrating tablets. J Pharm Innov. 2018;13(3):261–9. https://doi.org/10.1007/s12247-018-9323-3.
van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm. 2002;53(2):139–45. https://doi.org/10.1016/s0939-6411(01)00228-4.
Epe C, Kaminsky R. New advancement in anthelmintic drugs in veterinary medicine. Trends Parasitol. 2013;29(3):129–34. https://doi.org/10.1016/j.pt.2013.01.001.
Villar D, Cray C, Zaias J, Altman NH. Biologic effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci. 2007;46(6):8–15.
Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin, and coxibs) on the upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2010;24(2):121–32. https://doi.org/10.1016/j.bpg.2009.11.005.
Tian L, Bayen S, Yaylayan V. Thermal degradation of five veterinary and human pharmaceuticals using pyrolysis-GC/MS. J Anal Appl Pyrolysis. 2017;127:120–5. https://doi.org/10.1016/j.jaap.2017.08.016.
Ganzera M, Schneider P, Stuppner H. Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci. 2006;78(8):856–61. https://doi.org/10.1016/j.lfs.2005.05.095.
Acknowledgments
The authors acknowledge the Brazilian funding agencies DPI/UnB (Edital 04/2019), FAP-DF (193.001.741/2017), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). The authors would also like to thank CONICET/Argentina for providing the grants for GR Bedogni and the National University of Rosario (Argentina).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bedogni, G.R., Pires, F.Q., Chaker, J.A. et al. Elucidating the Splitting Behavior of Tablets to Optimize the Pharmacotherapy in Veterinary Medicine. AAPS PharmSciTech 22, 67 (2021). https://doi.org/10.1208/s12249-021-01937-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01937-8