Abstract
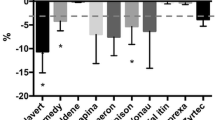
Tablet subdivision is a common practice used mainly for dose adjustment. The aim of this study was to investigate how the technical aspects of production as well as the method of tablets subdivision (employing a tablet splitter or a kitchen knife) influence the accuracy of this practice. Five drugs commonly used as subdivided tablets were selected. For each drug, the innovator drug product, a scored-generic and a non-scored generic were investigated totalizing fifteen drug products. Mechanical and physical tests, including image analysis, were performed. Additionally, comparisons were made between tablet subdivision method, score, shape, diluent composition and coating. Image analysis based on surface area was a useful tool as an alternative assay to evaluate the accuracy of tablet subdivision. The tablet splitter demonstrates an advantage relative to a knife as it showed better results in weight loss and friability tests. Oblong, coated and scored tablets had better results after subdivision than round, uncoated and non-scored tablets. The presence of elastic diluents such as starch and dibasic phosphate dehydrate conferred a more appropriate behaviour for the subdivision process than plastic materials such as microcrystalline cellulose and lactose. Finally, differences were observed between generics and their innovator products in all selected drugs with regard the quality control assays in divided tablet, which highlights the necessity of health regulations to consider subdivision performance at least in marketing authorization of generic products.






Similar content being viewed by others
REFERENCES
Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol. 2006;62:1065–73. doi:10.1007/s00228-006-0202-3.
Arruabarrena J, Coello J, Maspoch S. Raman spectroscopy as a complementary tool to assess the content uniformity of dosage units in break-scored warfarin tablets. Int J Pharm. 2014;465:299–305. doi:10.1016/j.ijpharm.2014.01.027.
Rodenhuis N, De Smet PAGM, Barends DM. The rationale of scored tablets as dosage form. Eur J Pharm Sci. 2004;21:305–8. doi:10.1016/j.ejps.2003.10.018.
Habib WA, Alanizi AS, Abdelhamid MM, Alanizi FK. Accuracy of tablet splitting: comparison study between hand splitting and tablet cutter. Saudi Pharm J. 2014;22:454–9. doi:10.1016/j.jsps.2013.12.014.
Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA. Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. AAPS PharmSciTech. 2010;11:1359–67. doi:10.1208/s12249-010-9515-8.
Weissman EM, Dellenbaugh C. Impact of splitting risperidone tablets on medication adherence and on clinical outcomes for patients with schizophrenia. Psychiatr Serv. 2007;58:201–6. doi:10.1176/appi.ps.58.2.201.
McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18:193–7. doi:10.1002/j.1875-9114.1998.tb03838.x.
Tahaineh LM, Gharaibeh SF. Tablet splitting and weight uniformity of half-tablets of four medications in pharmacy practice. J Pharm Pract. 2012;25:471–6. doi:10.1177/0897190012442716.
Elliott I, Mayxay M, Yeuichaixong S, Lee SJ, Newton PN. The practice and clinical implications of tablet splitting in international health. Trop Med Int Health. 2014;19:754–60. doi:10.1111/tmi.12309.
Marriott JL, Nation RL. Splitting tablets. Aust Prescr. 2002;25:133–5.
Mandal TK. Effect of tablet integrity on the dissolution rate of sustained-release preparations. J Clin Pharm Ther. 1996;21:155–7. doi:10.1023/A:1016442514205.
Fischbach MS, Gold JL, Lee M, Dergal JM, Litner GM, Rochon PA. Pill-splitting in a long-term care facility. JAMA. 2001;164:785–6.
Arnet I, Hersberger KE. Misleading score-lines on tablets: facilitated intake or fractional dosing? Swiss Med Wkly. 2010;140:105–10.
Teng J, Song CK, Williams RL, Polli JE. Lack of medication dose uniformity in commonly split tablets. J Am Pharm Assoc. 2002;42:195. doi:10.1331/108658002763508489.
Noviasky J, Lo V, Luft D. Which medications can be split without compromising efficacy and safety? J Fam Pract. 2006;55:707–8.
Ferreira AAA, Prates EC, Fernandes JPS, Ferrarini M. Avaliação do efeito da partição de comprimidos de furosemida sobre a uniformidade da dose. Rev Ciencias Farm Basica e Apl. 2011;32:47–53.
Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15:253–61.
Edwards S. Variability in tablet fragment weights when splitting unscored cyclobenzaprine 10 mg tablets. J Am Pharm Assoc. 2004;44:583. doi:10.1331/1544-3191.44.5.583.
Van Riet-Nales DA, Doeve ME, Nicia AE, Teerenstra S, Notenboom K, Hekster YA, et al. The accuracy, precision and sustainability of different techniques for tablet subdivision: breaking by hand and the use of tablet splitters or a kitchen knife. Int J Pharm. 2014;466:44–51. doi:10.1016/j.ijpharm.2014.02.031.
Pitt KG, Heasley MG. Determination of the tensile strength of elongated tablets. Powder Technol. 2013;238:169–75.
USP 2015. General chapters <1216> TABLET FRIABILITY, United States Phamacopeia, 2015.
FDA 2013. Guidance for industry—tablet scoring: nomenclature, labeling, and data for evaluation. U.S. Food and Drug Administration, 2013.
Lakio S, Vajna B, Farkas I, Salokangas H, Marosi G, Yliruusi J. Challenges in detecting magnesium stearate distribution in tablets. AAPS PharmSciTech. 2013;14:435–44. doi:10.1208/s12249-013-9927-3.
Uzunović A, Vranić E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn J Basic Med Sci. 2007;7:279–83.
Helmy SA. Tablet splitting: is it worthwhile? Analysis of drug content and weight uniformity for half tablets of 16 commonly used medications in the outpatient setting. J Manag Care Spec Pharm. 2015;21:76–86.
Gupta A, Hunt RL, Khan MA. Influence of tablet characteristics on weight variability and weight loss in split tablets. Am J Heal Pharm. 2008;65:2326–8. doi:10.2146/ajhp080371.
FDA 2014. U.S. Food and Drug Administration, understanding generic drugs, U.S. Food Drug Adm. 2014. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs. Accessed July 10, 2015.
Wilson MG, Kaiser FE, Morley JE. Tablet-breaking ability of older persons with type 2 diabetes mellitus. Diabetes Educ. 2001;27:530–40.
European Pharmacopoeia, European Directorate for the Quality of Medicines. Monograph 478 Tablets. 8.0 ed. 2016.
Murdoch D, McTavish D. Sertraline. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depression and obsessive-compulsive disorder. Drugs. 1992;44:604–24. doi:10.2165/00003495-199244040-00007.
Verrue C. Is splitting tablets dangerous? Nurs Times. 2011;107:23.
Boggie DT, DeLattre ML, Schaefer MG, Morreale AP, Plowman BK. Accuracy of splitting unscored valdecoxib tablets. Am J Heal Pharm. 2004;61:1482–3.
Van der Steen KC, Frijlink HW, Schipper CMA, Barends DM. Prediction of the ease of subdivision of scored tablets from their physical parameters. AAPS PharmSciTech. 2010;11:126–32. doi:10.1208/s12249-009-9365-4.
Fell JT, Rowe RC, Newton JM. The mechanical strength of film-coated tablets. J Pharm Pharmacol. 1979;31:69–72.
Sedrati M, Arnaud P, Fontan JE, Brion F. Splitting tablets in half. Am J Hosp Pharm. 1994;51:548–50.
Rojas J, Hernandez S. Effect of the compaction platform on the densification parameters of tableting excipients with different deformation mechanisms. Chem Pharm Bull. 2014;62:281–7. doi:10.1248/cpb.c13-00884.
Ayorinde JO, Itiola OA, Odeniyi MA. Effects of material properties and speed of compression on microbial survival and tensile strength in diclofenac tablet formulations. Arch Pharm Res. 2013;36:273–81. doi:10.1007/s12272-013-0027-4.
Ilić I, Govedarica B, Šibanc R, Dreu R, Srčič S. Deformation properties of pharmaceutical excipients determined using an in-die and out-die method. Int J Pharm. 2013;446:6–15. doi:10.1016/j.ijpharm.2013.02.001.
Rowe RC, Sheskey PJ, Quinn ME. Handbook of Pharmaceutical Excipients, 6th edn. Pharmaceutical Press, 2009.
ACKNOWLEDGMENTS
This research was supported by Brazilian agency FAP-DF project number 0193.001023/2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Teixeira, M.T., Sá-Barreto, L.C.L., Gratieri, T. et al. Key Technical Aspects Influencing the Accuracy of Tablet Subdivision. AAPS PharmSciTech 18, 1393–1401 (2017). https://doi.org/10.1208/s12249-016-0615-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0615-y