Abstract
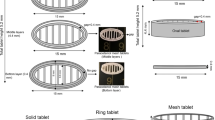
The objective of this work was to develop taste-masked donut-shaped tablet formulations utilizing fused filament fabrication three-dimensional printing paired with hot-melt extrusion techniques. Caffeine citrate was used as the model drug for its bitter taste, and a 3-point bend test was performed to assess the printability of filaments. The stiffness constant was calculated to represent the printability by fitting the breaking distances and stress data into Hooke’s law. The formulations without Eudragit E PO (F6) and with Eudragit E PO (F7) filaments exhibited the desired hardness with a “k” value of 48.30 ± 3.52 and 45.47 ± 3.51 g/mm3 (n = 10), respectively, and were successfully printed. The donut-shaped tablets were 3D printed with 10, 50, and 100% infill densities. In vitro dissolution studies were performed in simulated salivary fluid (pH 6.8, artificial saliva) to evaluate the taste-masking efficiency of the printed donuts. In the first minute, the concentrations of caffeine citrate observed in the dissolution media from all the printed donuts were less than the bitter threshold of caffeine citrate (0.25 mg/mL). Formulation F7, which contained Eudragit E PO copolymer, demonstrated better taste-masking efficiency than formulation F6. Furthermore, both formulations F6 and F7 demonstrated immediate drug release profiles in gastric medium (10% infill, > 80% release within 1 h). Taste-masked caffeine citrate formulations were successfully developed with donut shapes, which will enhance appeal in pediatric populations and increase compliance and patient acceptance of the dosage form.










Similar content being viewed by others
References
Maniruzzaman M, Boateng JS, Chowdhry BZ, Snowden MJ, Douroumis D. A review on the taste masking of bitter APIs: hot-melt extrusion (HME) evaluation. Drug Dev Ind Pharm. 2014;40(2):145–56. https://doi.org/10.3109/03639045.2013.804833.
Zheng JY, Keeney MP. Taste masking analysis in pharmaceutical formulation development using an electronic tongue. Int J Pharm. 2006;310(1–2):118–24. https://doi.org/10.1016/j.ijpharm.2005.11.046.
Tan DCT, Ong JJ, Gokhale R, Heng PWS. Hot melt extrusion of ion-exchange resin for taste masking. Int J Pharm. 2018;547(1–2):385–94. https://doi.org/10.1016/j.ijpharm.2018.05.068.
Petrovick GF, Breitkreutz J, Pein-Hackelbusch M. Taste-masking properties of solid lipid based micropellets obtained by cold extrusion-spheronization. Int J Pharm. 2016;506(1–2):361–70. https://doi.org/10.1016/j.ijpharm.2016.04.058.
Rachmawati H, Marbun EJ, Pamudji JS. Development of fast disintegrating tablet formula of ketoprofen-β-cyclodextrin inclusion complexes. Indian J Pharm. 2011;22(3):229–37. https://doi.org/10.14499/indonesianjpharm0iss0pp229-237.
Joshi S, Petereit HU. Film coatings for taste masking and moisture protection. Int J Pharm. 2013;457(2):395–406. https://doi.org/10.1016/j.ijpharm.2013.10.021.
Keating AV, Soto J, Tuleu C, Forbes C, Zhao M, Craig DQM. Solid state characterisation and taste masking efficiency evaluation of polymer based extrudates of isoniazid for paediatric administration. Int J Pharm. 2018;536(2):536–46. https://doi.org/10.1016/j.ijpharm.2017.07.008.
Gao Y, Cui FD, Guan Y, Yang L, Wang YS, Zhang LN. Preparation of roxithromycin-polymeric microspheres by the emulsion solvent diffusion method for taste masking. Int J Pharm. 2006;318(1–2):62–9. https://doi.org/10.1016/j.ijpharm.2006.03.018.
Petrovick GF, Kleinebudde P, Breitkreutz J. Orodispersible tablets containing taste-masked solid lipid pellets with metformin hydrochloride: influence of process parameters on tablet properties. Eur J Pharm Biopharm. 2018;122:137–45. https://doi.org/10.1016/j.ejpb.2017.10.018.
Khor CM, Ng WK, Kanaujia P, Chan KP, Dong Y. Hot-melt extrusion microencapsulation of quercetin for taste-masking. J Microencapsul. 2017;34(1):29–37. https://doi.org/10.1080/02652048.2017.1280095.
Nukala PK, Palekar S, Patki M, Patel K. Abuse deterrent immediate release egg-shaped tablet (Egglets) using 3D printing technology: quality by design to optimize drug release and extraction. AAPS PharmSciTech. 2019;20(2):80. https://doi.org/10.1208/s12249-019-1298-y.
Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–63. https://doi.org/10.1016/j.jconrel.2017.11.022.
Maniruzzaman M, Boateng JS, Bonnefille M, Aranyos A, Mitchell JC, Douroumis D. Taste masking of paracetamol by hot-melt extrusion: an in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80(2):433–42. https://doi.org/10.1016/j.ejpb.2011.10.019.
Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm. 2012;2012:436763–9. https://doi.org/10.5402/2012/436763.
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26. https://doi.org/10.1080/03639040701498759.
Reddy Dumpa N, Bandari S, Repka AR. Novel gastroretentive floating pulsatile drug delivery system produced via hot-melt extrusion and fused deposition modeling 3D printing. Pharmaceutics. 2020;12(1). https://doi.org/10.3390/pharmaceutics12010052.
Nober C, Manini G, Carlier E, Raquez JM, Benali S, Dubois P, et al. Feasibility study into the potential use of fused-deposition modeling to manufacture 3D-printed enteric capsules in compounding pharmacies. Int J Pharm. 2019;569:118581. https://doi.org/10.1016/j.ijpharm.2019.118581.
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. https://doi.org/10.1208/s12249-015-0360-7.
Dumpa NR, Sarabu S, Bandari S, Zhang F, Repka MA. Chronotherapeutic drug delivery of ketoprofen and ibuprofen for improved treatment of early morning stiffness in arthritis using hot-melt extrusion technology. AAPS PharmSciTech. 2018;19(6):2700–9. https://doi.org/10.1208/s12249-018-1095-z.
Juluri A, Popescu C, Zhou L, Murthy RN, Gowda VK, Chetan Kumar P, et al. Taste masking of griseofulvin and caffeine anhydrous using Kleptose Linecaps DE17 by hot melt extrusion. AAPS PharmSciTech. 2016;17(1):99–105. https://doi.org/10.1208/s12249-015-0374-1.
Tiwari RV, Polk AN, Patil H, Ye X, Pimparade MB, Repka MA. Rat palatability study for taste assessment of caffeine citrate formulation prepared via hot-melt extrusion technology. AAPS PharmSciTech. 2017;18(2):341–8. https://doi.org/10.1208/s12249-015-0447-1.
Lang B, McGinity JW, Williams RO 3rd. Hot-melt extrusion--basic principles and pharmaceutical applications. Drug Dev Ind Pharm. 2014;40(9):1133–55. https://doi.org/10.3109/03639045.2013.838577.
Arafat B, Qinna N, Cieszynska M, Forbes RT, Alhnan MA. Tailored on demand anti-coagulant dosing: an in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur J Pharm Biopharm. 2018;128:282–9. https://doi.org/10.1016/j.ejpb.2018.04.010.
Acosta-Velez FG. 3D pharming: direct printing of personalized pharmaceutical tablets. Polymer Science. 2016;2(1). https://doi.org/10.4172/2471-9935.100011.
Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, Markopoulou CK, Bouropoulos N, Tzetzis D, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur J Pharm Sci. 2018;120:40–52. https://doi.org/10.1016/j.ejps.2018.04.020.
Melocchi A, Parietti F, Loreti G, Maroni A, Gazzaniga A, Zema L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J Drug Deliv Sci Technol. 2015;30:360–7. https://doi.org/10.1016/j.jddst.2015.07.016.
Chai X, Chai H, Wang X, Yang J, Li J, Zhao Y, et al. Fused deposition modeling (FDM) 3D printed tablets for Intragastric floating delivery of Domperidone. Sci Rep. 2017;7(1):2829. https://doi.org/10.1038/s41598-017-03097-x.
Goyanes A, Chang H, Sedough D, Hatton GB, Wang J, Buanz A, et al. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int J Pharm. 2015;496(2):414–20. https://doi.org/10.1016/j.ijpharm.2015.10.039.
Skowyra J, Pietrzak K, Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–7. https://doi.org/10.1016/j.ejps.2014.11.009.
Li Q, Wen H, Jia D, Guan X, Pan H, Yang Y, et al. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharm. 2017;525(1):5–11. https://doi.org/10.1016/j.ijpharm.2017.03.066.
Zheng X, Wu F, Hong Y, Shen L, Lin X, Feng Y. Developments in taste-masking techniques for traditional Chinese medicines. Pharmaceutics. 2018;10(3). https://doi.org/10.3390/pharmaceutics10030157.
Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, et al. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur J Pharm Sci. 2018;118:191–9. https://doi.org/10.1016/j.ejps.2018.03.019.
Zhang J, Xu P, Vo AQ, Bandari S, Yang F, Durig T, et al. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J Drug Deliv Sci Technol. 2019;52:292–302. https://doi.org/10.1016/j.jddst.2019.04.043.
Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–97. https://doi.org/10.1016/j.ijpharm.2016.12.049.
Zhang J, Yang W, Vo AQ, Feng X, Ye X, Kim DW, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: structure and drug release correlation. Carbohydr Polym. 2017;177:49–57. https://doi.org/10.1016/j.carbpol.2017.08.058.
Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int J Pharm. 2016;499(1–2):376–94. https://doi.org/10.1016/j.ijpharm.2015.12.071.
Awad A, Trenfield SJ, Gaisford S, Basit AW. 3D printed medicines: a new branch of digital healthcare. Int J Pharm. 2018;548(1):586–96. https://doi.org/10.1016/j.ijpharm.2018.07.024.
Xu J, Bovet LL, Zhao K. Taste masking microspheres for orally disintegrating tablets. Int J Pharm. 2008;359(1–2):63–9. https://doi.org/10.1016/j.ijpharm.2008.03.019.
Alshehri SM, Park JB, Alsulays BB, Tiwari RV, Almutairy B, Alshetaili AS, et al. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J Drug Deliv Sci Technol. 2015;27:18–27. https://doi.org/10.1016/j.jddst.2015.03.003.
Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technologies. 2011;18(3):15–28. https://doi.org/10.14227/DT180311P15.
Bandari S, Dronam VR, Eedara BB. Development and preliminary characterization of levofloxacin pharmaceutical cocrystals for dissolution rate enhancement. J Pharm Investig. 2017;47(6):583–91. https://doi.org/10.1007/s40005-016-0302-8.
Pimparade MB, Morott JT, Park JB, Kulkarni VI, Majumdar S, Murthy SN, et al. Development of taste masked caffeine citrate formulations utilizing hot melt extrusion technology and in vitro-in vivo evaluations. Int J Pharm. 2015;487(1–2):167–76. https://doi.org/10.1016/j.ijpharm.2015.04.030.
Jeganathan B, Prakya V. Interpolyelectrolyte complexes of Eudragit® EPO with hypromellose acetate succinate and Eudragit® EPO with hypromellose phthalate as potential carriers for oral controlled drug delivery. AAPS PharmSciTech. 2015;16(4):878–88. https://doi.org/10.1208/s12249-014-0252-2.
Baishya H. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J Develop Drugs. 2017;06(02). https://doi.org/10.4172/2329-6631.1000171.
Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63. https://doi.org/10.1016/j.ijpharm.2015.04.069.
Abdelhakim HE, Coupe A, Tuleu C, Edirisinghe M, Craig DQM. Electrospinning optimization of Eudragit E PO with and without chlorpheniramine maleate using a design of experiment approach. Mol Pharm. 2019;16(6):2557–68. https://doi.org/10.1021/acs.molpharmaceut.9b00159.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9(1):250–8. https://doi.org/10.1208/s12249-008-9046-8.
Nokhodchi A, Raja S, Patel P, Asare-Addo K. The role of oral controlled release matrix tablets in drug delivery systems. Bioimpacts. 2012;2(4):175–87. https://doi.org/10.5681/bi.2012.027.
Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5(1):23–36. https://doi.org/10.1016/0168-3659(87)90034-4.
Funding
This project was also partially supported by Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Guest Editors: Feng Zhang, Michael Repka and Suresh Bandari
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Dumpa, N., Bandari, S. et al. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 21, 243 (2020). https://doi.org/10.1208/s12249-020-01783-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01783-0