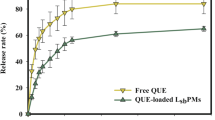
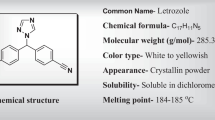
Abstract
Present investigation deals with formulation and evaluation of tamoxifen (TMX)-loaded liquid crystalline nanoparticles (TMX-LCNPs) for improving oral bioavailability and safety of the existing treatment. Hexagonal Glyceryl monooleate-based TMX-LCNPs (GLCNPs) and Phytantriol-based TMX-LCNPs (PLCNPs) were prepared by dilution-through-hydrotrope method for oral administration. Oleic acid was incorporated in the lipid matrix to enhance the drug loading in the LCNPs. Optimized LCNPs displayed small particle size with a narrow distribution, sustained drug release and high gastrointestinal stability. TMX-LCNPs were found to be considerably higher cytotoxic to MCF-7 cells as compared to free TMX. Substantial fold enhancement in oral bioavailability (~7- and ~5-folds with TMX-GLCNPs and TMX-PLCNPs, respectively) was evident followed by significant reduction in tumor burden with lesser hepatotoxicity. Out of the two LCNP formulations, PLCNPs were found to be better in convalescing the disease.






Similar content being viewed by others
References
Agrawal AK, Kumar K, Swarnakar NK, Kushwah V, Jain S. “Liquid crystalline nanoparticles”: rationally designed vehicle to improve stability and therapeutic efficacy of insulin following oral administration. Mol Pharm. 2017;14:1874–82.
Ayen WY, Garkhal K, Kumar N. Doxorubicin-loaded (PEG) 3-PLA nanopolymersomes: effect of solvents and process parameters on formulation development and in vitro study. Mol Pharm. 2011;8:466–78.
Barauskas J, Cervin C, Jankunec M, Špandyreva M, Ribokaitė K, Tiberg F, et al. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int J Pharm. 2010;391:284–91.
Barauskas J, Johnsson M, Tiberg F. Self-assembled lipid superstructures: beyond vesicles and liposomes. Nano Lett. 2005;5:1615–9.
Barbieri S, Buttini F, Rossi A, Bettini R, Colombo P, Ponchel G, et al. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int J Pharm. 2015;491:99–104.
Buchanan CM, Buchanan NL, Edgar KJ, Little JL, Malcolm MO, Ruble KM, et al. Pharmacokinetics of tamoxifen after intravenous and oral dosing of tamoxifen-hydroxybutenyl-beta-cyclodextrin formulations. J Pharm Sci. 2007;96:644–60.
Cho Y, Lee W, Choi J. Effects of curcumin on the pharmacokinetics of tamoxifen and its active metabolite, 4-hydroxytamoxifen, in rats: possible role of CYP3A4 and P-glycoprotein inhibition by curcumin. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2012;67:124–30.
Choi JS, Kang KW. Enhanced tamoxifen bioavailability after oral administration of tamoxifen in rats pretreated with naringin. Arch Pharm Res. 2008;31:1631–6.
Elefsiniotis IS, Pantazis KD, Ilias A, Pallis L, Mariolis A, Glynou I, et al. Tamoxifen induced hepatotoxicity in breast cancer patients with pre-existing liver steatosis: the role of glucose intolerance. Eur J Gastroenterol Hepatol. 2004;16:593–8.
Gao FF, Lv JW, Wang Y, Fan R, Li Q, Zhang Z, et al. Tamoxifen induces hepatotoxicity and changes to hepatocyte morphology at the early stage of endocrinotherapy in mice. Biomedical reports. 2016;4:102–6.
GreishK. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37.
Guo C, Wang J, Cao F, Lee RJ, Zhai G. Lyotropic liquid crystal systems in drug delivery. Drug Discov Today. 2010;15:1032–40.
Han F, Li S, Yin R, Liu H, Xu L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: nanostructured lipid carriers. Colloids Surf A Physicochem Eng Asp. 2008;315:210–6.
Hashem FM, Nasr M, Khairy A. In vitro cytotoxicity and bioavailability of solid lipid nanoparticles containing tamoxifen citrate. Pharm Dev Technol. 2014;19:824–32.
Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011b;32:503–15.
Jain AK, Thanki K, Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol Pharm. 2013;10:3459–74.
Jain AK, Thanki K, Jain S. Solidified self-nanoemulsifying formulation for oral delivery of combinatorial therapeutic regimen: part I. Formulation development, statistical optimization, and in vitro characterization. Pharm Res. 2014;31:923–45.
Jain S, Bhankur N, Swarnakar NK, Thanki K. Phytantriol based “stealth” lyotropic liquid crystalline nanoparticles for improved antitumor efficacy and reduced toxicity of docetaxel. Pharm Res. 2015;32:3282–92.
Jain S, Chauhan D, Jain A, Swarnakar N, Harde H, Mahajan R, et al. Stabilization of the nanodrug delivery systems by lyophilization using universal step-wise freeze drying cycle. Indian Patent Application No. 2559. 2011a.
Kapse SV, Gaikwad RV, Samad A, Devarajan PV. Self nanoprecipitating preconcentrate of tamoxifen citrate for enhanced bioavailability. Int J Pharm. 2012;429:104–12.
Kojima T, Katoh F, Matsuda Y, Teraoka R, Kitagawa S. Physicochemical properties of tamoxifen hemicitrate sesquihydrate. Int J Pharm. 2008;352:146–51.
Lai J, Lu Y, Yin Z, Hu F, Wu W. Pharmacokinetics and enhanced oral bioavailability in beagle dogs of cyclosporine encapsulated in glyceryl monooleate/poloxamer 407 cubic nanoparticles. Int J Nanomedicine. 2010;5:13–23.
Li C, Kim M, Choi H, Choi J. Effects of baicalein on the pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats: possible role of cytochrome P450 3A4 and P-glycoprotein inhibition by baicalein. Arch Pharm Res. 2011;34:1965–72.
Lian R, Lu Y, Qi J, Tan Y, Niu M, Guan P, et al. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. AAPS PharmSciTech. 2011;12:1234–40.
Lombardo D, Kiselev MA, Magazù S, Calandra P. Amphiphiles self-assembly: basic concepts and future perspectives of supramolecular approaches. Adv Condens Matter Phys. 2015. http://dx.doi.org/10.1155/2015/151683.
Nguyen T-H, Hanley T, Porter CJ, Boyd BJ. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J Control Release. 2011a;153:180–6.
Rizwan S, Hanley T, Boyd B, Rades T, Hook S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: characterisation, swelling and release kinetics. J Pharm Sci. 2009;98:4191–204.
Rossetti FC, Fantini MC, Carollo ARH, Tedesco AC, Lopes Badra Bentley MV. Analysis of liquid crystalline nanoparticles by small angle X‐ray diffraction: evaluation of drug and pharmaceutical additives influence on the internal structure. J Pharm Sci. 2011;100:2849–57.
Shi X, Peng T, Huang Y, Mei L, Gu Y, Huang J, et al. Comparative studies on glycerol monooleate-and phytantriol-based cubosomes containing oridonin in vitro and in vivo. Pharm Dev Technol. 2015;1–8.
Shin SC, Choi JS. Effects of epigallocatechin gallate on the oral bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Anti-Cancer Drugs. 2009;20:584–8.
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17:5748–56.
Swami, R., Singh, I., Jeengar, M.K., Naidu, V., Khan, W. & Sistla, R., 2015. Adenosine conjugated lipidic nanoparticles for enhanced tumor targeting. Int. J. Pharm, 486, 287–296.
Swarnakar NK, Jain V, Dubey V, Mishra D, Jain NK. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm Res. 2007;24:2223–30.
Swarnakar NK, Thanki K, Jain S. Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of Doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicity. Pharm Res. 2014a;31:1219–38.
Swarnakar NK, Thanki K, Jain S. Bicontinuous cubic liquid crystalline nanoparticles for oral delivery of Doxorubicin: implications on bioavailability, therapeutic efficacy, and cardiotoxicity. Pharm Res. 2014b;31:1219–38.
Swarnakar NK, Thanki K, Jain S. Lyotropic liquid crystalline nanoparticles of CoQ10: implication of lipase digestibility on oral bioavailability, in vivo antioxidant activity, and in vitro–in vivo relationships. Mol Pharm. 2014c;11:1435–49.
Yang Z, Tan Y, Chen M, Dian L, Shan Z, Peng X, et al. Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS PharmSciTech. 2012;13:1483–91.
Zeng N, Gao X, Hu Q, Song Q, Xia H, Liu Z, et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption. Int J Nanomedicine. 2012a;7:3703–18.
Zeng N, Gao X, Hu Q, Song Q, Xia H, Liu Z, et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption. Int J Nanomedicine. 2012b;7:3703–18.
Acknowledgments
The authors are thankful to Director, NIPER for providing the necessary infrastructure and facilities. R.S is grateful to Science and Engineering Research Board (SERB), DST, GOI, New Delhi, for providing research fellowship. VK is grateful to Council of Scientific and Industrial Research (CSIR), GOI, New Delhi for providing fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Jain, S., Heeralal, B., Swami, R. et al. Improved Oral Bioavailability, Therapeutic Efficacy, and Reduced Toxicity of Tamoxifen-Loaded Liquid Crystalline Nanoparticles. AAPS PharmSciTech 19, 460–469 (2018). https://doi.org/10.1208/s12249-017-0851-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-017-0851-9