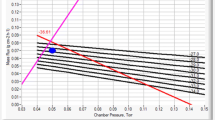
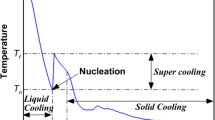
Abstract
Lyophilization is an approach commonly undertaken to formulate drugs that are unstable to be commercialized as ready to use (RTU) solutions. One of the important aspects of commercializing a lyophilized product is to transfer the process parameters that are developed in lab scale lyophilizer to commercial scale without a loss in product quality. This process is often accomplished by costly engineering runs or through an iterative process at the commercial scale. Here, we are highlighting a combination of computational and experimental approach to predict commercial process parameters for the primary drying phase of lyophilization. Heat and mass transfer coefficients are determined experimentally either by manometric temperature measurement (MTM) or sublimation tests and used as inputs for the finite element model (FEM)-based software called PASSAGE, which computes various primary drying parameters such as primary drying time and product temperature. The heat and mass transfer coefficients will vary at different lyophilization scales; hence, we present an approach to use appropriate factors while scaling-up from lab scale to commercial scale. As a result, one can predict commercial scale primary drying time based on these parameters. Additionally, the model-based approach presented in this study provides a process to monitor pharmaceutical product robustness and accidental process deviations during Lyophilization to support commercial supply chain continuity. The approach presented here provides a robust lyophilization scale-up strategy; and because of the simple and minimalistic approach, it will also be less capital intensive path with minimal use of expensive drug substance/active material.






Similar content being viewed by others
References
Day, J.G. and G.N. Stacey, eds. Methods in Molecular Biology. Cryopreservation and Freeze-Drying Protocols. 2007;368:153–161.
McLellan MR, Day JG. Cryopreservation and freeze-drying protocols. Introduction. Methods Mol Biol. 1995;38:1–5.
Hibler S, Gieseler H. Heat transfer characteristics of current primary packaging systems for pharmaceutical freeze-drying. J Pharm Sci. 2012;101(11):4025–31.
Kramer T et al. A procedure to optimize scale-up for the primary drying phase of lyophilization. J Pharm Sci. 2009;98(1):307–18.
Patel SM, Doen T, Pikal MJ. Determination of end point of primary drying in freeze-drying process control. AAPS PharmSciTech. 2010;11(1):73–84.
Schwegman JJ, Hardwick LM, Akers MJ. Practical formulation and process development of freeze-dried products. Pharm Dev Technol. 2005;10(2):151–73.
Gieseler H, Kramer T, Pikal MJ. Use of manometric temperature measurement (MTM) and SMART freeze dryer technology for development of an optimized freeze-drying cycle. J Pharm Sci. 2007;96(12):3402–18.
Gieseler H et al. Evaluation of tunable diode laser absorption spectroscopy for in-process water vapor mass flux measurements during freeze drying. J Pharm Sci. 2007;96(7):1776–93.
Kuu WY, Nail SL. Rapid freeze-drying cycle optimization using computer programs developed based on heat and mass transfer models and facilitated by tunable diode laser absorption spectroscopy (TDLAS). J Pharm Sci. 2009;98(9):3469–82.
Tang XC, Nail SL, Pikal MJ. Evaluation of manometric temperature measurement (MTM), a process analytical technology tool in freeze drying, part III: heat and mass transfer measurement. AAPS PharmSciTech. 2006;7(4):97.
Tang X, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200.
Tang XC, Nail SL, Pikal MJ. Freeze-drying process design by manometric temperature measurement: design of a smart freeze-dryer. Pharm Res. 2005;22(4):685–700.
Johnson RE et al. Use of manometric temperature measurements (MTM) to characterize the freeze-drying behavior of amorphous protein formulations. J Pharm Sci. 2010;99(6):2863–73.
Milton N et al. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J Pharm Sci Technol. 1997;51(1):7–16.
Pikal MJ et al. The nonsteady state modeling of freeze drying: in-process product temperature and moisture content mapping and pharmaceutical product quality applications. Pharm Dev Technol. 2005;10(1):17–32.
Pikal MJ. Use of laboratory data in freeze drying process design: heat and mass transfer coefficients and the computer simulation of freeze drying. J Parenter Sci Technol. 1985;39(3):115–39.
Koganti VR et al. Investigation of design space for freeze-drying: use of modeling for primary drying segment of a freeze-drying cycle. AAPS PharmSciTech. 2011;12(3):854–61.
Rambhatla S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, I: atypical radiation and the edge vial effect. AAPS PharmSciTech. 2003;4(2):E14.
Rambhatla S, Tchessalov S, Pikal MJ. Heat and mass transfer scale-up issues during freeze-drying, III: control and characterization of dryer differences via operational qualification tests. AAPS PharmSciTech. 2006;7(2):E39.
Mascarenhas WJ, Akay HU, Pikal MJ. A computational model for finite element analysis of the freeze-drying process. Comput Methods Appl Mech Eng. 1997;148:105–24.
Mockus LN et al. Quality by design in formulation and process development for a freeze-dried, small molecule parenteral product: a case study. Pharm Dev Technol. 2011;16(6):549–76.
Pisano R et al. Quality by design: scale-up of freeze-drying cycles in pharmaceutical industry. AAPS PharmSciTech. 2013;14(3):1137–49.
Nail SL. The effect of chamber pressure on heat transfer in the freeze drying of parenteral solutions. J Parenter Drug Assoc. 1980;34(5):358–68.
Schneid SC, Gieseler H. Rational approaches and transfer strategies for the scale-up of freeze-drying cycles. Process Dev. 2011;29(1):43–7.
Acknowledgments
This work was undertaken in the BMS Drug Product Science & Technology at New Brunswick, and support from Dr. Rajesh Gandhi and Dr. John Crison is gratefully acknowledged. The authors would like to thank the helpful discussion with Dr. Henning Gieseler and Yan Liu of Technalysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, X., Sadineni, V., Maity, M. et al. Finite Element Method (FEM) Modeling of Freeze-drying: Monitoring Pharmaceutical Product Robustness During Lyophilization. AAPS PharmSciTech 16, 1317–1326 (2015). https://doi.org/10.1208/s12249-015-0318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0318-9