No Heading
Purpose.
To develop a procedure based on manometric temperature measurement (MTM) and an “expert system” for good practices in freeze drying that will allow development of an optimized freeze-drying process during a single laboratory freeze-drying experiment.
Methods.
Freeze drying was performed with a FTS Dura-Stop/Dura-Top freeze dryer with the manometric temperature measurement software installed. Five percent solutions of glycine, sucrose, or mannitol with 2 ml to 4 ml fill in 5 ml vials were used, with all vials loaded on one shelf. Details of freezing, optimization of chamber pressure, target product temperature, and some aspects of secondary drying are determined by the expert system algorithms. MTM measurements were used to select the optimum shelf temperature, to determine drying end points, and to evaluate residual moisture content in real-time. MTM measurements were made at 1 hour or half-hour intervals during primary drying and secondary drying, with a data collection frequency of 4 points per second. The improved MTM equations were fit to pressure-time data generated by the MTM procedure using Microcal Origin software to obtain product temperature and dry layer resistance. Using heat and mass transfer theory, the MTM results were used to evaluate mass and heat transfer rates and to estimate the shelf temperature required to maintain the target product temperature.
Results.
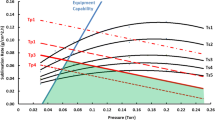
MTM product dry layer resistance is accurate until about two-thirds of total primary drying time is over, and the MTM product temperature is normally accurate almost to the end of primary drying provided that effective thermal shielding is used in the freeze-drying process. The primary drying times can be accurately estimated from mass transfer rates calculated very early in the run, and we find the target product temperature can be achieved and maintained with only a few adjustments of shelf temperature. The freeze-dryer overload conditions can be estimated by calculation of heat/mass flow at the target product temperature. It was found that the MTM results serve as an excellent indicator of the end point of primary drying. Further, we find that the rate of water desorption during secondary drying may be accurately measured by a variation of the basic MTM procedure. Thus, both the end point of secondary drying and real-time residual moisture may be obtained during secondary drying.
Conclusions.
Manometric temperature measurement and the expert system for good practices in freeze drying does allow development of an optimized freeze-drying process during a single laboratory freeze-drying experiment.
Similar content being viewed by others
References
1. M. J. Pikal. Lyophilization. In J. Warbrick and J. C. Boylan (eds.), Marcel Dekker, New York, NY. pp. 1299–1326 (2002).
2. M. J. Pikal. Freeze-drying of proteins. Part I: process design. BioPharm 3:18–20, 22–24, 26–28 (1990).
3. A. P. Mackenzie. Basic principles of freeze-drying for pharmaceuticals. Bull. Parenter. Drug Assoc. 20:101–130 (1966).
4. A. I. Liapis and J. M. Marchello. Advances in the modeling and control of freeze-drying, Adv. Drying 3:217–244 (1984).
5. M. J. Pikal. Use of laboratory data in freeze drying process design: heat and mass transfer coefficients and the computer simulation of freeze drying. J. Parenter. Sci. Technol. 39:115–139 (1985).
6. N. Milton, M. J. Pikal, M. L. Roy, and S. L. Nail. Evaluation of manometric temperature measurement as a method of monitoring product temperature during lyophilization. PDA J. Pharm. Sci. Technol. 51:7–16 (1997).
7. X. C. Tang, S. L. Nail, and M. J. Pikal. Evaluation of manometric temperature measurement (mtm) in freeze drying part I: product temperature measurement. Pharm. Sci. Tech. (in press).
8. X. C. Tang, S. L. Nail, and M. J. Pikal. Evaluation of manometric temperature measurement (MTM) in freeze drying part II: measurement of dry layer resistance. Pharm. Sci. Tech. (in press).
9. X. C. Tang, S. L. Nail, and M. J. Pikal. Evaluation of manometric temperature measurement (MTM) in freeze drying part III: heat transfer coefficient measurement. Pharm. Sci. Tech. (in press).
10. M. J. Pikal, M. L. Roy, and S. Shah. Mass and heat transfer in vial freeze-drying of pharmaceuticals: role of the vial. J. Pharm. Sci. 73:1224–1237 (1984).
11. G. Jansco, J. Pupezin, and W. A. Van Hook. The vapor pressure of ice between 0.01 and −100 C. J. Phys. Chem. 74:2984–2989 (1970).
12. L. Chang, X. Tang, M. J. Pikal, N. Milton, and L. Thomas. The origin of multiple glass transitions in frozen aqueous solutions, Proceedings of the NATAS Annual Conference on Thermal Analysis, Vol. 27, 1999, pp. 624–628.
13. M. J. Pikal, S. Shah, D. Senior, and J. E. Lang. Physical chemistry of freeze-drying: measurement of sublimation rates for frozen aqueous solutions by a microbalance technique. J. Pharm. Sci. 72:635–650 (1983).
14. X. C. Tang and M. J. Pikal. Design of freeze drying processes for pharmaceuticals: practical advice. Pharm. Res. 21:191–200 (2004).
15. M. L. Roy and M. J. Pikal. Process control in freeze drying: determination of the end point of sublimation drying by an electronic moisture sensor. J. Parenter. Sci. Technol. 43:60–66 (1989).
16. G.-W. Oetjen. Industrial freeze-drying for pharmaceutical applications. Drugs and the Pharmaceutical Sciences 96:267–335 (1999).
17. X. C. Tang, S. L. Nail, and M. J. Pikal. Evaluation of manometric temperature measurement (MTM) in freeze drying part IV: the impact of water re-absorption. Pharm. Sci. Tech. (in press).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, X., Nail, S. & Pikal, M. Freeze-Drying Process Design by Manometric Temperature Measurement: Design of a Smart Freeze-Dryer. Pharm Res 22, 685–700 (2005). https://doi.org/10.1007/s11095-005-2501-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2501-2