Abstract
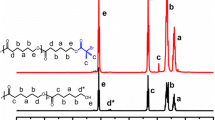
The clinical application of amphotericin B (AmB), a broad spectrum antifungal agent, is limited by its poor solubility in aqueous medium and also by its proven renal toxicity. In this work, AmB was encapsulated in micelles obtained from the self-assembly of PDMAEMA-b-PCL-b-PDMAEMA triblock copolymers. The amount of encapsulated AmB depended on the copolymer composition, and short blocks of polycaprolactone (PCL) and poly(2-dimethylaminoethyl methacrylate) (PDMAEMA) showed better performance. All the studied formulations exhibited a controlled release of AmB along 150 h. The formulations presented reduced hemotoxicity while maintaining antifungal activities against Candida albicans, Candida krusei, and Candida glabrata comparable with free AmB. A reduction on the hemotoxicity was found to be due to the slow release and subsequent low aggregation achieved with the use of polymer micelle nanocontainers.









Similar content being viewed by others
References
Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–29.
Hamill R. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013;73:919–34.
Groll A, Walsh T. Uncommon opportunistic fungi: new nosocomial threats. Clin Microbiol Infect. 2001;7:8–24.
Deray G. Amphotericin B, nephrotoxicity. J Antimicrob Chemother. 2002;49:37–41.
Legrand P, Romero EA, Cohen BE, Bolard J. Effects of aggregation and solvent on the toxicity of amphotericin B to human erythrocytes. Antimicrob Agents Chemother. 1992;36:2518–22.
Ringdén O, Meunier F, Tollemar J, Ricci P, Tura S, Kuse E, et al. Efficacy of amphotericin B encapsulated in liposomes (AmBisome) in the treatment of invasive fungal infections in immunocompromised patients. J Antimicrob Chemother. 1991;28:73–82.
Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764–71.
Alexandridis P, Lindman B. Amphiphilic block copolymers: self-assembly and applications. Amsterdam: Elsevier; 2000.
Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73:137–72.
Loh XJ, Wu Y-L, Joseph Seow WT, Irzuan Norimzan MN, Zhang Z-X, Xu F-J, et al. Micellization and phase transition behavior of thermosensitive poly (N-isopropylacrylamide)–poly (ɛ-caprolactone)–poly (N-isopropylacrylamide) triblock copolymers. Polymer. 2008;49:5084–94.
Cabral H, Kataoka K. Progress of drug-loaded polymeric micelles into clinical studies. J Control Release. 2014;190:465–76.
Pippa N, Mariaki M, Pispas S, Demetzos C. Preparation, development and in vitro release evaluation of amphotericin B-loaded amphiphilic block copolymer vectors. Int J Pharm. 2014;473:80–6.
Yoon H-J, Jang W-D. Polymeric supramolecular systems for drug delivery. J Mater Chem. 2010;20:211–22.
Falamarzian A, Lavasanifar A. Chemical modification of hydrophobic block in poly(ethylene oxide) poly(caprolactone) based nanocarriers: effect on the solubilization and hemolytic activity of amphotericin B. Macromol Biosci. 2010;10:648–56.
Adams ML, Andes DR, Kwon GS. Amphotericin B encapsulated in micelles based on poly(ethylene oxide)-block-poly(L-amino acid) derivatives exerts reduced in vitro hemolysis but maintains potent in vivo antifungal activity. Biomacromolecules. 2003;4:750–7.
Yang ZL, Yang KW, Li XR, Liu Y. Preparation and in vitro release kinetics of amphotericin B-loaded poly (ethyleneglycol)-poly (dl-lactide) block copolymer micelles. Chin Pharm J. 2007;42:519–23.
Adams ML, Kwon GS. Relative aggregation state and hemolytic activity of amphotericin B encapsulated by poly(ethylene oxide)-block–poly(N-hexyl-l-aspartamide)-acyl conjugate micelles: effects of acyl chain length. J Control Release. 2003;87:23–32.
Sinha V, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 2004;278:1–23.
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32:762–98.
Gaucher G, Dufresne M-H, Sant VP, Kang N, Maysinger D, Leroux J-C. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109:169–88.
Burt HM, Zhang X, Toleikis P, Embree L, Hunter WL. Development of copolymers of poly (D, L-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf B: Biointerfaces. 1999;16:161–71.
Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly (ε-caprolactone) and poly (ethylene glycol) for doxorubicin delivery. J Control Release. 2004;98:415–26.
Aliabadi HM, Elhasi S, Mahmud A, Gulamhusein R, Mahdipoor P, Lavasanifar A. Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: the effect of solvent composition on micellar properties and drug loading. Int J Pharm. 2007;329:158–65.
Xu F, Neoh K, Kang E. Bioactive surfaces and biomaterials via atom transfer radical polymerization. Prog Polym Sci. 2009;34:719–61.
Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J, et al. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials. 2014;35:2322–35.
Lee AS, Gast AP, Bütün V, Armes SP. Characterizing the structure of pH dependent polyelectrolyte block copolymer micelles. Macromolecules. 1999;32:4302–10.
Baines F, Billingham N, Armes S. Synthesis and solution properties of water-soluble hydrophilic-hydrophobic block copolymers. Macromolecules. 1996;29:3416–20.
Liu S, Weaver JV, Tang Y, Billingham NC, Armes SP, Tribe K. Synthesis of shell cross-linked micelles with pH-responsive cores using ABC triblock copolymers. Macromolecules. 2002;35:6121–31.
Zhang W, He J, Liu Z, Ni P, Zhu X. Biocompatible and pH-responsive triblock copolymer mPEG-b-PCL-b-PDMAEMA: synthesis, self-assembly, and application. J Polym Sci A Polym Chem. 2010;48:1079–91.
Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv Drug Deliv Rev. 2002;54:169–90.
Lebouille JJL, Vleugels LW, Dias A, Leermakers FM, Cohen Stuart M, Tuinier R. Controlled block copolymer micelle formation for encapsulation of hydrophobic ingredients. Eur Phys J E. 2013;36:1–12.
McLaughlin CK, Logie J, Shoichet MS. Core and corona modifications for the design of polymeric micelle drug-delivery systems. Isr J Chem. 2013;53:670–9.
Diaz I, Perez L. Synthesis and micellization properties of triblock copolymers PDMAEMA-b-PCL-b-PDMAEMA and their applications in the fabrication of amphotericin B-loaded nanocontainers. Colloid Polym Sci. 2014;1–11.
Maiti S, Chatterji PR, Nisha C, Manorama S, Aswal VK, Goyal PS. Aggregation and polymerization of PEG-based macromonomers with methacryloyl group as the only hydrophobic segment. J Colloid Interface Sci. 2001;240:630–5.
Shim WS, Kim SW, Choi EK, Park HJ, Kim JS, Lee DS. Novel pH sensitive block copolymer micelles for solvent free drug loading. Macromol Biosci. 2006;6:179–86.
Rao JP, Geckeler KE. Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci. 2011;36:887–913.
Wang C-H, Wang W-T, Hsiue G-H. Development of polyion complex micelles for encapsulating and delivering amphotericin B. Biomaterials. 2009;30:3352–8.
Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharm Res. 2002;19:418–22.
Rojas J, García A, López A. Evaluación de dos metodologías para determinar la actividad antimicrobiana de plantas medicinales. Bol Latinoam Caribe Plant Med Aromat. 2005;4:28–32.
Luján MC, Pérez Corral C. Cribado para evaluar actividad antibacteriana y antimicótica en plantas utilizadas en medicina popular de Argentina. Rev Cuba Farm 2008;42
Fittler A, Kocsis B, Gerlinger I, Botz L. Optimization of bioassay method for the quantitative microbiological determination of amphotericin B. Mycoses. 2010;53:57–61.
Allen C, Maysinger D, Eisenberg A. Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf B: Biointerfaces. 1999;16:3–27.
Shim YH, Lee HJ, Dubois P, Chung CW, Jeong YI. Amphotericin B aggregation inhibition with novel nanoparticles prepared with poly (ε-caprolactone)/poly (N, N-dimethylamino-2-ethyl methacrylate) diblock copolymer. J Microbiol Biotechnol. 2011;21:28–36.
Mok MM, Thiagarajan R, Flores M, Morse DC, Lodge TP. Apparent critical micelle concentrations in block copolymer/ionic liquid solutions: remarkably weak dependence on solvophobic block molecular weight. Macromolecules. 2012;45:4818–29.
Stoodley R, Wasan KM, Bizzotto D. Fluorescence of amphotericin B-deoxycholate (Fungizone) monomers and aggregates and the effect of heat-treatment. Langmuir. 2007;23:8718–25.
Barwicz J, Gruszecki WI, Gruda I. Spontaneous organization of amphotericin B in aqueous medium. J Colloid Interface Sci. 1993;158:71–6.
Jain JP, Kumar N. Development of amphotericin B loaded polymersomes based on (PEG)3-PLA co-polymers: factors affecting size and in vitro evaluation. Eur J Pharm Sci. 2010;40:456–65.
Ahmad Z, Shah A, Siddiq M, Kraatz H-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4:17028–38.
Yu BG, Okano T, Kataoka K, Sardari S, Kwon GS. In vitro dissociation of antifungal efficacy and toxicity for amphotericin B-loaded poly(ethylene oxide)-block-poly(β-benzyl-L-aspartate) micelles. J Control Release. 1998;56:285–91.
Pujol I, Pastor FJ, dos Santos Lazéra M, Artigas JG. Evaluation of the Neo-Sensitabs® diffusion method for determining the antifungal susceptibilities of Cryptococcus gattii isolates, using three different agar media. Rev Iberoam Micol. 2008;25:215–20.
Ruiz HK, Serrano DR, Dea-Ayuela MA, Bilbao-Ramos PE, Bolás-Fernández F, Torrado JJ, et al. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int J Pharm. 2014;473:148–57.
ACKNOWLEDGEMENTS
The authors thank to the Pontificia Universidad Javeriana for the financial support throught the grant number 5620.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diaz, I.L., Parra, C., Linarez, M. et al. Design of Micelle Nanocontainers Based on PDMAEMA-b-PCL-b-PDMAEMA Triblock Copolymers for the Encapsulation of Amphotericin B. AAPS PharmSciTech 16, 1069–1078 (2015). https://doi.org/10.1208/s12249-015-0298-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-015-0298-9