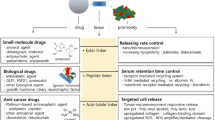
Abstract
Through many years of clinical application of long-acting injectables, there is clear proof that this type of formulation does not just provide the patient with convenience, but more importantly a more effective treatment of the medication provided. The formulation approach therefore contains huge untapped potential to improve the quality of life of many patients with a variety of different diseases. This review provides a summary of some of the central talks provided at the workshop with focus on aqueous suspensions and their use as a long-acting injectable. Elements as formulation, manufacturing, in vitro dissolution methods, in vitro and in vivo correlation, in silico modelling provide an insight into some of the current understandings, learnings, and not least gaps in the field.
Graphical Abstract









Similar content being viewed by others
References
Burgess DJ, Wright JC. An introduction to long acting injections and implants. In: In Burgess DJ, Wright JC, editors. long acting injections and implants. Springer; 2012. p. 1–9.
Kipp JE. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm. 2004;284:109–22.
Rhee YS, Park CW, Deluca PP, Mansour HM. Sustained-release injectable drug delivery: a review of current and future systems. PharmTechnol. 2010;s6:8–13.
Maresova P, Javanmardi E, Barakovic S, BarakovicHusic J, Tomsone S, Krejcar O, Kuca K. Consequences of chronic diseases and other limitations associated with old age - a scoping review. BMC Public Health. 2019;19:1431.
Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4:198–219.
Remenar JF. Making the leap from daily oral dosing to long-acting injectables: lessons from the antipsychotics. Mol Pharm. 2014;11:1739–49.
Rubio JM, Schoretsanitis G, John M, Tiihonen J, Taipale H, Guinart D, Malhotra AK, Correll CU, Kane JM. Psychosis relapse during treatment with long-acting injectable antipsychotics in individuals with schizophrenia-spectrum disorders: an individual participant data meta-analysis. Lancet Psychiatry. 2020;7:749–61.
Morris MT, Tarpada SP. Long-acting injectable paliperidone palmitate: A review of efficacy and safety. Psychopharmacol Bull. 2017;47:42–52.
Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv Drug Deliv Rev. 2016;103:144–56.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96.
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Therapeut. 2001;23:1296–310.
Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, Alam T. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18:527–39.
Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012;28:669–80.
Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;15:e22-33.
Leenen FH, Wilson TW, Bolli P, Larochelle P, Myers M, Handa SP, Boileau G, Tanner J. Patterns of compliance with once versus twice daily antihypertensive drug therapy in primary care: a randomized clinical trial using electronic monitoring. Can J Cardiol. 1997;13:914–20.
Eberlin M, Otto G, Krämer I. Increased medication compliance of liver transplant patients switched from a twice-daily to a once-daily tacrolimus-based immunosuppressive regimen. Transplant Proc. 2013;45:2314–20.
Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55:886–91.
Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67:453–60.
Sajatovic M, Levin J, Ramirez LF, Hahn DY, Tatsuoka C, Bialko CS, Cassidy KA, Fuentes-Casiano E, Williams TD. Prospective trial of customized adherence enhancement plus long-acting injectable antipsychotic medication in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2013;74:1249–55.
Morrissette DA, Stahl SM. Optimizing outcomes in schizophrenia: long-acting depots and long-term treatment. CNS Spectr. 2012;17(Suppl 1):10–21.
Okoli CTC, Kappi A, Wang T, Makowski A, Cooley AT. The effect of long-acting injectable antipsychotic medications compared with oral antipsychotic medications among people with schizophrenia: A systematic review and meta-analysis. Int J Ment Health Nurs. 2022;31:469–535.
Hirsch SR, Gaind R, Rohde PD, Stevens BC, Wing JK. Outpatient maintenance of chronic schizophrenic patients with long-acting fluphenazine: double-blind placebo trial. Report to the Medical Research Council Committee on Clinical Trials in Psychiatry. Br Med J. 1973;1:633–7.
Kaunitz AM. Injectable long-acting contraceptives. Clin Obstet Gynecol. 2001;44:73–91.
Swindells S, Siccardi M, Barrett SE, Olsen DB, Grobler JA, Podany AT, Nuermberger E, Kim P, Barry CE, Owen A, Hazuda D, Flexner C. Long-acting formulations for the treatment of latent tuberculous infection: opportunities and challenges. Int J Tuberc Lung Dis. 2018;22:125–32.
FDA. 2023; https://www.fda.gov/drugs/human-immunodeficiency-virus-hiv/fda-approves-cabenuva-and-vocabria-treatment-hiv-1-infection. accessed 13-Feb-2023.
Giliad. 2023; https://www.gilead.com/news-and-press/press-room/press-releases/2021/3/gilead-and-merck-announce-agreement-to-jointly-develop-and-commercialize-longacting-investigational-treatment-combinations-of-lenacapavir-and-islatr. accessed 13-Feb-2023.
Liversidge GG, Cundy KC, Bishop JF, Czekai DA. Surface Modified Drug Nanoparticles. United States Patent. 1992;5:145684.
Shi J, Wang D, Tian Y, Wang Z, Gao J, Liu N, Gao X, Zheng A, Zhang H, Xiang M. Comparison of paliperidone palmitate from different crystallization processes and effect on formulations in vitro and in vivo. Pharmaceutics. 2022;14:1094.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53.
Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–69.
Kesisoglou F, Panmai S, Wu Y. Nanosizing–oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–44.
Malamatari M, Taylor KMG, Malamataris S, Douroumis D, Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23:534–47.
Li M, Azad M, Davé R, Bilgili E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics. 2016;8:17.
Verma S, Kumar S, Gokhale R, Burgess DJ. Physical stability of nanosuspensions: investigation of the role of stabilizers on Ostwald ripening. Int J Pharm. 2011;406:145–52.
Thanh NT, Maclean N, Mahiddine S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev. 2014;114:7610–30.
Nyqvist H, Wadsten T. Structure-related variation in epimer ratio and solvent content of bacampicillin hydrochloride. Acta Pharm Suec. 1985;22:215–28.
De Cleyn E, Holm R, Van den Mooter G. Shedding a light on the physical stability of suspensions micronised with intensified vibratory milling; A trend observed with decreasing particle size as a function of time. Int J Pharm. 2021;603:120687.
Joshi V, Dwivedi S, Ward GH. Increase in the specific surface area of budesonide during storage postmicronization. Pharm Res. 2002;19:7–12.
Lee RW, Shaw JM, McShane J, Wood RW, Shenoy DB. Particle size reduction. In: Water-insoluble drug formulations, Liu R (editor), Interpharm Press, 2008, Second Edition, chapter 17, pp. 467–99.
Kesteleyn B, Amssoms K, Schepens W, Hache G, Verschueren W, Van De Vreken W, Rombauts K, Meurs G, Sterkens P, Stoops B, Baert L, Austin N, Wegner J, Masungi C, Dierynck I, Lundgren S, Jönsson D, Parkes K, Kalayanov G, Wallberg H, Rosenquist A, Samuelsson B, Van Emelen K, Thuring JW. Design and synthesis of HIV-1 protease inhibitors for a long-acting injectable drug application. Bioorg Med Chem Lett. 2013;23:310–7.
Netzsch. 2022. (https://grinding.netzsch.com/en/pharma-cosmetics/wet-grinding), accessed 10-Nov-2022.
WAB. 2022. (https://www.wab-group.com/en/grinding-dispersing/agitator-bead-mills/) , accessed 10-Nov-2022.
Lee RW. Case study: Development and scale-up of nanocrystal® particles. In: Injectable dispersed systems: formulation, processing and performance, Burgess DJ (editor), Boca Raton; Taylor & Francis, 2005, chapter 10, pp. 355–70.
FDA document of Invega Sustanna. Url: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022264s000clinpharmr.pdf., accessed 10-Nov-2022.
Bao Q, Wang X, Zou Y, Wang Y, Burgess DJ. In vitro release testing method development for long-acting injectable suspensions. Int J Pharm. 2022;622:121840.
Bao Q, Jog R, Shen J, Newman B, Wang Y, Choi S, Burgess DJ. Physicochemical attributes and dissolution testing of ophthalmic ointments. Int J Pharm. 2017;523:310–9.
Xu X, Al-Ghabeish M, Rahman Z, Krishnaiah YS, Yerlikaya F, Yang Y, Manda P, Hunt RL, Khan MA. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int J Pharm. 2015;493:412–25.
Bao Q, Shen J, Jog R, Zhang C, Newman B, Wang Y, Choi S, Burgess DJ. In vitro release testing method development for ophthalmic ointments. Int J Pharm. 2017;526:145–56.
Bao Q, Wang X, Zou Y, Wang Y, Burgess DJ. In vitro release testing method development for long-acting injectable suspensions. Int J Pharm. 2022;622:121840.
Andhariya JV, Shen J, Choi S, Wang Y, Zou Y, Burgess DJ. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J Control Release. 2017;255:27–35.
Wan B, Bao Q, Burgess DJ. In vitro-in vivo correlation of PLGA microspheres: Effect of polymer source variation and temperature. J Control Release. 2022;347:347–55.
Wan B, Bao Q, Wang R, Burgess DJ. Polymer source affects in vitro-in vivo correlation of leuprolide acetate PLGA microspheres. Int J Pharm. 2022;625:122032.
Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12.
Bassand C, Villois A, Gianola L, Laue G, Ramazani F, Riebesehl B, Sanchez-Felix M, Sedo K, Ullrich T, Duvnjak RM. Smart design of patient-centric long-acting products: from preclinical to marketed pipeline trends and opportunities. Expert Opin Drug Deliv. 2022;19:1265–83.
Dubbelboer IR, Sjögren E. Physiological based pharmacokinetic and biopharmaceutics modelling of subcutaneously administered compounds - An overview of in silico models. Int J Pharm. 2022;621:121808.
Darville N, van Heerden M, Mariën D, DeMeulder M, Rossenu S, Vermeulen A, Vynckier A, De Jonghe S, Sterkens P, Annaert P, Van den Mooter G. The effect of macrophage and angiogenesis inhibition on the drug release and absorption from an intramuscular sustained-release paliperidone palmitate suspension. J Control Release. 2016;230:95–108.
Utembe W, Clewell H, Sanabria N, Doganis P, Gulumian M. Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials. Nanomaterials. 2020;10:1267.
Jucker BM, Alsaid H, Rambo M, Lenhard SC, Hoang B, Xie F, Groseclose MR, Castellino S, Damian V, Bowers G, Gupta M. Multimodal imaging approach to examine biodistribution kinetics of cabotegravir (GSK1265744) long acting parenteral formulation in rat. J Control Release. 2017;268:102–12.
Paquette SM, Dawit H, Hickey MB, Merisko-Liversidge E, Almarsson O, Deaver DR. Long-acting atypical antipsychotics: Characterization of the local tissue response. Pharm Res. 2014;31:2065–77.
Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, Piscitelli S. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67:481–6.
Acknowledgements
We would like to thank Mrs. Zahra Ghaemmaghamian for her help with formatting the figures.
Funding
Diane J. Burgess and Quanying Bao received a U.S. Food and Drug Administration grant (HHSF223201710135C).
Author information
Authors and Affiliations
Contributions
All authors have contributed to the conception of the work, each have drafted individual sections and commented all sections of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
R,L., J.G., and N.D. are employees of Lubrizol Life Science, V.L. is an employee and holds stock of Simulations Plus and S.A. is an employee of GlaxoSmithKline. The authors report no other conflicts of interest in in this work.
Additional information
Communicated by Huybrecht T'jollyn and Oliver Ackaert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Holm, R., Lee, R.W., Glassco, J. et al. Long-Acting Injectable Aqueous Suspensions—Summary From an AAPS Workshop. AAPS J 25, 49 (2023). https://doi.org/10.1208/s12248-023-00811-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-023-00811-8