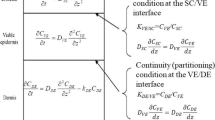
Abstract
A combined experimental and computational model approach was developed to assess heat effects on drug delivery from transdermal delivery systems (TDSs) in vitro and nicotine was the model drug. A Franz diffusion cell system was modified to allow close control of skin temperature when heat was applied from an infrared lamp in vitro. The effects of different heat application regimens on nicotine fluxes from two commercial TDSs across human cadaver skin were determined. Results were interpreted in terms of transport parameters estimated using a computational heat and mass transport model. Steady-state skin surface temperature was obtained rapidly after heat application. Increasing skin surface temperature from 32 to 42°C resulted in an approximately 2-fold increase in average nicotine flux for both TDSs, with maximum flux observed during early heat application. ANOVA statistical analyses of the in vitro permeation data identified TDS differences, further evidenced by the need for a two-layer model to describe one of the TDSs. Activation energies associated with these data suggest similar temperature effects on nicotine transport across the skin despite TDS design differences. Model simulations based on data obtained from continuous heat application were able to predict system response to intermittent heat application, as shown by the agreement between the simulation results and experimental data of nicotine fluxes under four different heat application regimens. The combination of in vitro permeation testing and a computational model provided a parameter-based heat and mass transport approach to evaluate heat effects on nicotine TDS delivery.







Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CHADD:
-
Controlled heat-assisted drug delivery
- C :
-
Concentration
- c adh :
-
Fast-release compartmental concentration in the two-layer TDS
- C 0 :
-
TDS concentration
- C max :
-
Maximum concentration
- DE:
-
Dermis
- D adh :
-
Fast-release compartmental diffusivity in the two-layer TDS
- D p :
-
Homogeneous TDS diffusivity
- D p2 :
-
Slow-release compartmental diffusivity in the two-layer TDS
- D SC_32 :
-
Stratum corneum diffusivity (DSC) for 32°C
- D SC_42 :
-
DSC for 42°C
- E A :
-
Activation energy
- IVPT:
-
In vitro permeation test
- J max :
-
Maximum (peak) flux
- J max_sim :
-
Simulation Jmax
- J max_exp :
-
Experimental Jmax
- K SCp :
-
SC/TDS partition coefficient
- K oct :
-
Octanol-water partition coefficient
- M f :
-
Total (cumulative) amount of drug permeated
- M(t):
-
Cumulative amount of drug permeated at a given time
- PBS:
-
Phosphate-buffered saline
- PID:
-
Proportional-integral-derivative
- SC:
-
Stratum corneum
- T:
-
Temperature
- t max :
-
Time when maximum flux occurs
- t max_sim :
-
Simulation tmax
- TDS:
-
Transdermal delivery system(s)
- UC/UB:
-
University of Cincinnati/University of Buffalo
- VE:
-
Viable epidermis
- z :
-
Position in the membrane along the axis perpendicular to the skin surface
References
Scheuplein RJ. Analysis for permeability data for the case of parallel diffusion pathways. Biophys J. 1966;6:1–17.
Potts RO, Francoeur ML. Lipid biophysics of water loss through the skin. Proc Natl Acad Sci U S A. 1990;87:3871–3.
Blank IH, Scheuplein RJ, MacFarlane DJ. Mechanism of percutaneous absorption III. The effect of temperature on the transport of non-electrolytes across the skin. J Invest Dermatol. 1967;49:582–9.
Peck KD, Ghanem A-H, Higuchi WI. The effect of temperature upon the permeation of polar and ionic solutes through human epidermal membrane. J Pharm Sci. 1995;84:975–82.
Tominaga K, Tojo K. Effect of environmental temperature on transdermal drug penetration. Biol Pharm Bull. 2010;33:1983–7.
Oliveira G, Leverett JC, Emamzadeh M, Lane ME. The effect of heat on skin barrier function and in vivo dermal absorption. Int J Pharm. 2014;464:145–51.
Akomeah F, Nazir T, Martin GP, Brown MB. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004;21:337–45.
Clarys P, Alewaeters K, Jadoul N, Barel A, Manadas RO, Preat V. In vitro percutaneous penetration through hairless rat skin: influence of temperature, vehicle, and penetration enhancers. Eur J Pharm Biopharm. 1998;46:279–83.
Wood DG, Brown MB, Jones SA. Understanding heat-facilitated drug transport across human epidermis. Eur J Pharm Biopharm. 2012;81:642–9.
Park J-H, Lee J-W, Kim Y-C, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. 2008;359:94–103.
Hull W. Enhanced transdermal delivery: a survey paper. J Appl Res. 2002;2:1–10.
Prodduturi S, Sadrieh N, Wokovich AM, Doub WH, Westenberger BJ, Buhse L. Transdermal delivery of fentanyl from matrix and reservoir systems: effect of heat and compromised skin. J Pharm Sci. 2010;99:2357–66.
Varvel JR, Shafer SL, Hwang SS, Coen PA, Stanski DR. Absorption characteristics of transdermally administered fentanyl. Anesthesiol. 1989;70:928–34.
Ashburn MA, Ogden LL, Zhang J, Love G, Basta SV. The pharmacokinetics of transdermal fentanyl delivered with and without controlled heat. J Pain. 2003;4:291–7.
Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011;3:236–42.
Klemsdal TO, Gjesdal K, Bredesen J-E. Heating and cooling of the nitroglycerin patch application area modify the plasma level of nitroglycerin. Eur J Clin Pharmacol. 1992;43:625–8.
Moore KJ, Sathyan G, Richarz U, Natarajan J, Vandenbossche J. Randomized 5-treatment crossover study to assess the effects of external heat on serum fentanyl concentrations during treatment with transdermal fentanyl systems. J Clin Pharmacol. 2012;52:1174–85.
Shomaker TS, Zhang J, Ashburn MA. Assessing the impact of heat on the systemic delivery of fentanyl through the transdermal fentanyl delivery system. Pain Med. 2000;1:225–30.
Vanakoski J, Seppala T, Sievi E, Lunell E. Exposure to high ambient temperature increases absorption and plasma concentrations of transdermal nicotine. Clin Pharmacol Ther. 1996;60:308–15.
Gupta SK, Southam M, Hwang SS. System functionality and physicochemical model of fentanyl transdermal system. J Pain Symptom Manage. 1992;7:S17–26.
Gourlay GK. Treatment of cancer pain with transdermal fentanyl. Lancet Oncol. 2001;2:165–72.
Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5:230–41.
Rose PG, Macfee MS, Boswell MV. Fentanyl transdermal system overdose secondary to cutaneous hyperthermia. Anesth Analg. 1993;77:390–1.
Snackey K. Transdermal fentanyl patches and heat-associated toxicities. Hospital U. The Prescription. 4. San Antonio, TX: University Health System Pharmacy Dept; 2007.
Hao J, Ghosh P, Li SK, Newman B, Raney SG, Kasting GB, et al. Heat effects on drug delivery across human skin. Expert Opin Drug Deliv. 2016;13:755–68.
La Count TD, Zhang Q, Hao J, Ghosh P, Raney SG, Talattof A, et al. Modeling temperature-dependent dermal absorption and clearance for transdermal and topical drug applications. AAPS J 22(3):70. https://doi.org/10.1208/s12248-020-00451-2.
Shin SH, Thomas S, Raney SG, Ghosh P, Hammell DC, El-Kamary SS, et al. In vitro-in vivo correlations for nicotine transdermal delivery systems evaluated by both in vitro skin permeation (IVPT) and in vivo serum pharmacokinetics under the influence of transient heat application. J Control Release. 2018;270:76–88.
Kasting GB, Bowman LA. DC electrical properties of frozen, excised human skin. Pharm Res. 1990;7:134–43.
Zhang Q, Murawsky M, La Count TD, Hao J, Kasting GB, Newman B, et al. Characterization of temperature profiles in skin and transdermal delivery system when exposed to temperature gradients in vivo and in vitro. Pharm Res. 2017;34:1491–504.
EPA. Estimation Program Interface (EPI) Suite v 4.11 Washington DC: U.S. Environmental Protection Agency; 2013 [Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.
Widmer RJ, Stewart RH, Young MF, Laurinec JE, Laine GA, Quick CM. Application of local heat induces capillary recruitment in the Pallid bat wing. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2312–7.
Dancik Y, Miller M, Jaworska J, Kasting GB. Design and performance of a spreadsheet-based model for estimating bioavailability of chemicals from dermal exposures. Adv Drug Deliv Rev. 2013;65:221–36.
Mitragotri S. Temperature dependence of skin permeability to hydrophilic and hydrophobic solutes. J Pharm Sci. 2007;96:1832–9.
Brown MB, Traynor MJ, Martin GP, Akomeah FK. Transdermal drug delivery systems: skin perturbation devices. In: Jain KK, editor. Drug Delivery Systems. Totowa NJ: Humana Press (Springer Science); 2008.
Cohen ML. Measurement of the thermal properties of human skin. A review. J Invest Dermatol. 1977;69:333–8.
Scheuplein RJ. Mechanism of percutaneous absorption II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol. 1967;48:79–88.
Li X, Johnson R, Weinstein B, Wilder E, Smith E, Kasting GB. Dynamics of water transport and swelling in human stratum corneum. Chem Eng Sci. 2015;138:164–72.
Acknowledgments
The authors gratefully acknowledge the contributions to the IVPT study design by Dr. Audra Stinchcomb, Dr. Hazem Hassan, Soo Hyeon Shin, and others at the University of Maryland, as well as Dr. Bryan Newman, Dr. Robert Lionberger, and others at the FDA. The authors also thank Daniel M. Frey for his help in the laboratory.
Funding
Funding for this project was made possible, in part, by the US Food and Drug Administration (FDA) through a cooperative agreement (Research Award U01 FD004942). In response to funding opportunity announcement RFA-FD-13-015, separate research projects were awarded in parallel to the University of Cincinnati and the University of Maryland, and each institution was requested by the FDA to perform independent research with the same drug products under comparable study conditions in a manner coordinated by the FDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
This article reflects the views of the authors and should not be construed to represent the US Food and Drug Administration’s views or policies. The views expressed in this paper do not reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Figure S1
Experimental (symbols) and simulated (curve) cumulative amount data of nicotine permeation across human skin from TDS-N2 at 32°C for a single diffusion cell. (GIF 242 kb)
Supplementary Figure S2.
Representative temperature versus time plot. Insert depicts expanded time scale showing initial application of heat and PID controller adjusting heat to set value. (GIF 261 kb)
Supplementary Figure S3
Optimized (fitted) TDS-N1 flux from three models (homogeneous TDS, two-layer TDS, and adjusted two-layer TDS) in comparison to experimental flux data (symbols) at baseline and elevated temperatures, which shows the adjusted two-layer TDS model best characterizes TDS-N1 drug delivery. Heat application was for 24 h for the 42°C case (arrows). (GIF 564 kb)
Supplementary Figure S4
In vitro release test (IVRT) of nicotine release from TDS-N1 (circle symbols) and TDS-N2 (square symbols) at 32°C (open symbols) and 42°C (closed symbols) in Franz diffusion cell using a filter membrane (without skin). Heat was applied from 0-24 h for the 42°C case (arrows). Mean ± SEM, n = 11–12. (GIF 128 kb)
Supplementary Figure S5
Representative image of the release liner (left), internal membrane (middle), and backing (right) of TDS-N1. (GIF 1546 kb)
ESM 6
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
La Count, T.D., Zhang, Q., Murawsky, M. et al. Evaluation of Heat Effects on Transdermal Nicotine Delivery In Vitro and In Silico Using Heat-Enhanced Transport Model Analysis. AAPS J 22, 82 (2020). https://doi.org/10.1208/s12248-020-00457-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-020-00457-w