Abstract
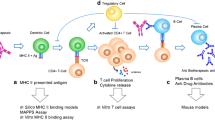
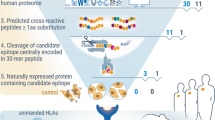
Most immune responses to biotherapeutic proteins involve the development of anti-drug antibodies (ADAs). New drugs must undergo immunogenicity assessments to identify potential risks at early stages in the drug development process. This immune response is T cell-dependent. Ex vivo assays that monitor T cell proliferation often are used to assess immunogenicity risk. Such assays can be expensive and time-consuming to carry out. Furthermore, T cell proliferation requires presentation of the immunogenic epitope by major histocompatibility complex class II (MHCII) proteins on antigen-presenting cells. The MHC proteins are the most diverse in the human genome. Thus, obtaining cells from subjects that reflect the distribution of the different MHCII proteins in the human population can be challenging. The allelic frequencies of MHCII proteins differ among subpopulations, and understanding the potential immunogenicity risks would thus require generation of datasets for specific subpopulations involving complex subject recruitment. We developed TCPro, a computational tool that predicts the temporal dynamics of T cell counts in common ex vivo assays for drug immunogenicity. Using TCPro, we can test virtual pools of subjects based on MHCII frequencies and estimate immunogenicity risks for different populations. It also provides rapid and inexpensive initial screens for new biotherapeutics and can be used to determine the potential immunogenicity risk of new sequences introduced while bioengineering proteins. We validated TCPro using an experimental immunogenicity dataset, making predictions on the population-based immunogenicity risk of 15 protein-based biotherapeutics. Immunogenicity rankings generated using TCPro are consistent with the reported clinical experience with these therapeutics.





Similar content being viewed by others
References
Joubert MK, Deshpande M, Yang J, Reynolds H, Bryson C, Fogg M, et al.. Use of in vitro assays to assess immunogenicity risk of antibody-based biotherapeutics. PLoS One. 2016;11(8):e0159328.
Ridker PM, Tardif J-C, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al.. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. 2017;376(16):1517–26.
Mahlangu J, Weldingh K, Lentz S, Kaicker S, Karim F, Matsushita T, et al. Changes in the amino acid sequence of the recombinant human factor VIIa analog, vatreptacog alfa, are associated with clinical immunogenicity. J Thromb Haemost. 2015;13(11):1989–98.
Wang Y-MC, Wang J, Hon YY, Zhou L, Fang L, Ahn HY. Evaluating and reporting the immunogenicity impacts for biological products—a clinical pharmacology perspective. AAPS J. 2016;18(2):395–403.
Svenningsson A, Dring AM, Fogdell-Hahn A, Jones I, Engdahl E, Lundkvist M, et al. Fatal neuroinflammation in a case of multiple sclerosis with anti-natalizumab antibodies. Neurology. 2013;80(10):965–7.
DeFrancesco L. Three deaths sink Affymax: Nature Publishing Group; 2013.
Vultaggio A, Matucci A, Nencini F, Pratesi S, Parronchi P, Rossi O, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy. 2010;65(5):657–61.
Srivastava A, Brewer A, Mauser-Bunschoten E, Key N, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47.
Hoffman M, Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J Thromb Haemost. 2012;10(8):1478–85.
D'arcy CA, Mannik M. Serum sickness secondary to treatment with the murine–human chimeric antibody IDEC-C2B8 (rituximab). Arthritis Rheum. 2001;44(7):1717–8.
D'Angiolella L, Cortesi P, Rocino A, Coppola A, Hassan H, Giampaolo A, et al. The socio-economic burden of patients affected by hemophilia with inhibitors. Eur J Haematol. 2018;101:435–56.
Mahlangu J, Paz P, Hardtke M, Aswad F, Schroeder J. TRUST trial: BAY 86-6150 use in haemophilia with inhibitors and assessment for immunogenicity. Haemophilia. 2016;22(6):873–9.
Kotarek J, Stuart C, De Paoli SH, Simak J, Lin T-L, Gao Y, et al. Subvisible particle content, formulation, and dose of an erythropoietin peptide mimetic product are associated with severe adverse postmarketing events. J Pharm Sci. 2016;105(3):1023–7.
Lamberth K, Weldingh KN, Ehrenforth S, Chéhadé MR, Østergaard H. Immunogenicity lessons learned from the clinical development of vatreptacog alfa, a recombinant activated factor VII analog, in Hemophilia with inhibitors. Protein Therapeutics: Springer; 2017. p. 123–60.
Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61.
Rosenberg AS, Sauna ZE. Immunogenicity assessment during the development of protein therapeutics. J Pharm Pharmacol. 2017.
Bachelet D, Hässler S, Mbogning C, Link J, Ryner M, Ramanujam R, et al. Occurrence of anti-drug antibodies against interferon-beta and natalizumab in multiple sclerosis: a collaborative cohort analysis. PLoS One. 2016;11(11):e0162752.
Wullner D, Zhou L, Bramhall E, Kuck A, Goletz TJ, Swanson S, et al. Considerations for optimization and validation of an in vitro PBMC derived T cell assay for immunogenicity prediction of biotherapeutics. Clin Immunol. 2010;137(1):5–14.
Schultz HS, Reedtz-Runge SL, Bäckström BT, Lamberth K, Pedersen CR, Kvarnhammar AM. Quantitative analysis of the CD4+ T cell response to therapeutic antibodies in healthy donors using a novel T cell: PBMC assay. PLoS One. 2017;12(5):e0178544.
Zubler RH, editor Naive and memory B cells in T-cell-dependent and T-independent responses. Springer seminars in immunopathology. Springer; 2001.
Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: preclinical assessment and mitigation. Clin Immunol. 2013;149(3):534–55.
Baker M, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self/nonself. 2010;1(4):314–22.
La Gruta NL, Gras S, Daley SR, Thomas PG, Rossjohn J. Understanding the drivers of MHC restriction of T cell receptors. Nat Rev Immunol. 2018;1.
Robinson J, Waller MJ, Parham P, Groot ND, Bontrop R, Kennedy LJ, et al. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31(1):311–4.
Jensen KK, Andreatta M, Marcatili P, Buus S, Greenbaum JA, Yan Z, et al. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology. 2018.
Baker MP, Jones TD. Identification and removal of immunogenicity in therapeutic proteins. Curr Opin Drug Discov Dev. 2007;10(2):219–27.
Gourraud P-A, Khankhanian P, Cereb N, Yang SY, Feolo M, Maiers M, et al. HLA diversity in the 1000 genomes dataset. PLoS One. 2014;9(7):e97282.
Karle A, Spindeldreher S, Kolbinger F, editors. Secukinumab, a novel anti–IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. MAbs. Taylor & Francis; 2016.
Ritter G, Cohen LS, Williams C, Richards EC, Old LJ, Welt S. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res. 2001;61(18):6851–9.
Welt S, Ritter G, Williams C, Cohen LS, Jungbluth A, Richards EA, et al. Preliminary report of a phase I study of combination chemotherapy and humanized A33 antibody immunotherapy in patients with advanced colorectal cancer. Clin Cancer Res. 2003;9(4):1347–53.
Scott AM, Lee F-T, Jones R, Hopkins W, MacGregor D, Cebon JS, et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin Cancer Res. 2005;11(13):4810–7.
Delluc S, Ravot G, Maillere B. Quantitative analysis of the CD4 T-cell repertoire specific to therapeutic antibodies in healthy donors. FASEB J. 2011;25(6):2040–8.
Gordon M, Margolin K, Talpaz M, Sledge G Jr, Holmgren E, Benjamin R, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19(3):843–50.
Tajima N, Martinez A, Kobayashi F, He L, Dewland P. A phase 1 study comparing the proposed biosimilar BS-503a with bevacizumab in healthy male volunteers. Pharmacol Res Perspect. 2017;5(2).
Rubic-Schneider T, Kuwana M, Christen B, Aßenmacher M, Hainzl O, Zimmermann F, et al. T-cell assays confirm immunogenicity of tungsten-induced erythropoietin aggregates associated with pure red cell aplasia. 2017;1(6):367–79.
Delluc S, Ravot G, Maillere B. Quantification of the pre-existing CD4 T cell repertoire specific for human erythropoietin reveals its immunogenicity potential. Blood. 2010:blood-2010-04-280875.
Casadevall N, Dobronravov V, Eckardt K-U, Ertürk S, Martynyuk L, Schmitt S, et al. Evaluation of the safety and immunogenicity of subcutaneous HX575 epoetin alfa in the treatment of anemia associated with chronic kidney disease in predialysis and dialysis patients. Clin Nephrol. 2017;88(4):190–7.
Shin S-K, Moon SJ, Ha SK, Jo Y-I, Lee T-W, Lee YS, et al. Immunogenicity of recombinant human erythropoietin in Korea: a two-year cross-sectional study. Biologicals. 2012;40(4):254–61.
Fineman M, Mace K, Diamant M, Darsow T, Cirincione B, Booker Porter T, et al. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab. 2012;14(6):546–54.
Milicevic Z, Anglin G, Harper K, Konrad R, Skrivanek Z, Glaesner W, et al. Low incidence of anti-drug antibodies in patients with type 2 diabetes treated with once-weekly glucagon-like peptide-1 receptor agonist dulaglutide. Diabetes Obes Metab. 2016;18(5):533–6.
Meunier S, Menier C, Marcon E, Lacroix-Desmazes S, Maillère B. CD4 T cells specific for factor VIII are present at high frequency in healthy donors and comprise naïve and memory cells. Blood Adv. 2017;1(21):1842–7.
Iorio A, Fischer K, Makris M. Large scale studies assessing anti-factor VIII antibody development in previously untreated haemophilia A: what has been learned, what to believe and how to learn more. Br J Haematol. 2017;178(1):20–31.
Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim S-B, et al. Subcutaneous versus intravenous administration of (neo) adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–78.
Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639.
Pivot X, Bondarenko I, Nowecki Z, Dvorkin M, Trishkina E, Ahn J-H, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2–positive early breast cancer. J Clin Oncol. 2018;36(10):968–74.
Spindeldreher S. Comparison of T cell assays: results from the ABIRISK consortium. 9th Open EIP Scientific Symposium And Final ABIRISK Open conference on Immunogenicity of Biopharmaceuticals. Lisbon, Portugal; 2017.
Spindeldreher S, Maillère B, Correia E, Tenon M, Karle A, Jarvis P, et al. Secukinumab demonstrates significantly lower immunogenicity potential compared to ixekizumab. Dermatol Ther. 2018;8(1):57–68.
Ara-Martín M, Pinto PH, Pascual-Salcedo D. Impact of immunogenicity on response to anti-TNF therapy in moderate-to-severe plaque psoriasis: results of the PREDIR study. J Dermatol Treat. 2017;28(7):606–12.
Benucci M, Gobbi FL, Meacci F, Manfredi M, Infantino M, Severino M, et al. Antidrug antibodies against TNF-blocking agents: correlations between disease activity, hypersensitivity reactions, and different classes of immunoglobulins. Biol Targets Ther. 2015;9:7.
Reyes-Beltrán B, Delgado G. Anti-drug antibodies in Colombian patients with rheumatoid arthritis treated with Enbrel vs Etanar–preliminary report. J Immunotoxicol. 2017;14(1):103–8.
Plasencia C, Pascual-Salcedo D, Nuño L, Bonilla G, Villalba A, Peiteado D, et al. Influence of immunogenicity on the efficacy of long-term treatment of spondyloarthritis with infliximab. Ann Rheum Dis. 2012:annrheumdis-2011-200828.
Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology. 2011;50(8):1445–52.
Baert F, Noman M, Vermeire S, Van Assche G, D'haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–8.
Cohen SB, Alten R, Kameda H, Hala T, Radominski SC, Rehman MI, et al. A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy. Arthritis Res Ther. 2018;20(1):155.
Hanauer S. Safety of infliximab in clinical trials. Aliment Pharmacol Ther. 1999;13:16–22.
Reich K, Jackson K, Ball S, Garces S, Kerr L, Chua L, et al. Ixekizumab pharmacokinetics, anti-drug antibodies, and efficacy through 60 weeks of treatment of moderate to severe plaque psoriasis. J Investig Dermatol. 2018;138(10):2168–73.
Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab for active Crohn’s disease. N Engl J Med. 2003;348(1):24–32.
Lundkvist M, Engdahl E, Holmen C, Movérare R, Olsson T, Hillert J, et al. Characterization of anti-natalizumab antibodies in multiple sclerosis patients. Mult Scler J. 2013;19(6):757–64.
van Vollenhoven RF, Emery P, Bingham CO, Keystone EC, Fleischmann R, Furst DE, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010:jrheum. 090856.
Piro L, White C, Grillo-Lopez A, Janakiraman N, Saven A, Beck T, et al. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1999;10(6):655–61.
Reich K, Blauvelt A, Armstrong A, Langley R, Fox T, Huang J, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176(3):752–8.
Deodhar AA, Gladman DD, McInnes IB, Strand V, Ren M, Spindeldreher S, et al. Secukinumab immunogenicity in patients with psoriatic arthritis and ankylosing spondylitis during a 52-week treatment period. Arthritis Rheumatol. 2018.
Adedokun OJ, Xu Z, Gasink C, Jacobstein D, Szapary P, Johanns J, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154(6):1660–71.
Gokemeijer J, Jawa V, Mitra-Kaushik S. How close are we to profiling immunogenicity risk using in silico algorithms and in vitro methods?: an industry perspective. AAPS J. 2017:1–6.
Swaminathan A, Lucas RM, Dear K, McMichael AJ. Keyhole limpet haemocyanin–a model antigen for human immunotoxicological studies. Br J Clin Pharmacol. 2014;78(5):1135–42.
Inaba K, Metlay JP, Crowley MT, Witmer-Pack M, Steinman RM. Dendritic cells as antigen presenting cells in vivo. Int Rev Immunol. 1990;6(2–3):197–206.
Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152(6):2675–85.
Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719.
Charron L, Doctrinal A, Ni Choileain S, Astier AL. Monocyte: T-cell interaction regulates human T-cell activation through a CD28/CD46 crosstalk. Immunol Cell Biol. 2015;93(9):796–803.
Gorbet MB, Sefton MV. Endotoxin: the uninvited guest. Biomaterials. 2005;26(34):6811–7.
Ryan J. Endotoxins and cell culture. Corning Life Sciences Technical Bulletin. 2004;1–8.
Münz C, Steinman RM, Fujii S-I. Dendritic cell maturation by innate lymphocytes. J Exp Med. 2005;202(2):203–7.
Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells:“l'union fait la force”. Blood. 2005;106(7):2252–8.
Milo R. What is the total number of protein molecules per cell volume? A call to rethink some published values. Bioessays. 2013;35(12):1050–5.
Chen X, Hickling TP, Vicini P. A mechanistic, multiscale mathematical model of immunogenicity for therapeutic proteins: part 1—theoretical model. CPT Pharmacometrics Syst Pharmacol. 2014;3(9):e133.
Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Investig. 2006;116(9):2423–33.
Squibb B-M. Opdivo (nivolumab) package insert. Princeton: Bristol-Myers Squibb; 2015.
Dhanda SK, Grifoni A, Pham J, Vaughan K, Sidney J, Peters B, et al. Development of a strategy and computational application to select candidate protein analogues with reduced HLA binding and immunogenicity. Immunology. 2018;153(1):118–32.
Chen X, Hickling T, Vicini P. A mechanistic, multiscale mathematical model of immunogenicity for therapeutic proteins: part 2—model applications. CPT Pharmacometrics Syst Pharmacol. 2014;3(9):1–10.
Acknowledgments
This project was supported in part by an appointment to the Research Participation Program at CBER, US Food and Drug Administration, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and FDA.
Author information
Authors and Affiliations
Contributions
Conceptualization: ONY, ZES, JRM, MAT, HY.
Data curation: ONY, ZES, JRM.
Formal analysis: ONY.
Funding acquisition: HY, ZES.
Investigation: ONY, ZES.
Methodology: ONY, ZES, JRM, MAT, HY.
Project administration: HY, ZES, MAT.
Resources: HY, ZES.
Software: ONY.
Supervision: HY, ZES, MAT.
Validation: ONY.
Visualization: ONY.
Writing—original draft: ONY, ZES, JRM.
Writing—review and editing: ONY, ZES, JRM, MAT, HY.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA's views or policies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 696 kb)
Rights and permissions
About this article
Cite this article
Yogurtcu, O.N., Sauna, Z.E., McGill, J.R. et al. TCPro: an In Silico Risk Assessment Tool for Biotherapeutic Protein Immunogenicity. AAPS J 21, 96 (2019). https://doi.org/10.1208/s12248-019-0368-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0368-0