Abstract
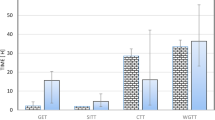
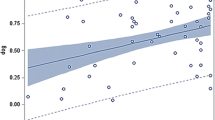
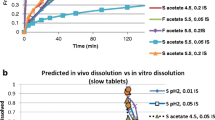
The extrapolation of oral bioavailability (F) information between dogs and humans has had an important role in the drug development process, whether it be to support an assessment of potential human pharmaceutical formulations or to identify the bioavailability challenges that may be encountered in dogs. Accordingly, these interspecies extrapolations could benefit from a tool that helps identify those drug characteristics consistent with species similarities in F. Our initial effort to find such a tool led to an exploration of species differences as it pertained to the biopharmaceutics classification system (BCS). However, using a range of compounds, we concluded that solubility and permeability alone could not explain interspecies inconsistencies in estimates of F. Therefore, we have now extended our evaluation to include canine versus human comparisons of F based upon the biopharmaceutics drug disposition classification system (BDDCS) and the extended clearance classification system (ECCS). Using the same data as that in our initial BCS assessments, we conclude that although neither the BDDCS nor the ECCS can reliably improve our ability to determine when F will be similar in humans and dogs, the ECCS provides a mechanism to help define possible causes for observed human-canine inconsistencies.





Similar content being viewed by others
References
Papich MG, Martinez MN. Applying biopharmaceutical classification system (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J. 2015;17(4):948–64.
Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20.
Lin L, Wong H. Predicting oral drug absorption: mini review on physiologically-based pharmacokinetic models. Pharmaceutics. 2017; 9(4). doi: https://doi.org/10.3390/pharmaceutics9040041.
Dahlgren D, Roos C, Johansson P, Lundqvist A, Tannergren C, Abrahamsson B, et al. Regional intestinal permeability in dogs: biopharmaceutical aspects for development of oral modified-release dosage forms. Mol Pharm. 2016;13(9):3022–33.
Dressman JB. Comparison of canine and human gastrointestinal physiology. Pharm Res. 1986;3(3):123–31.
Koziolek M, Grimm M, Bollmann T, Schäfer KJ, Blattner SM, Lotz R, et al. Characterization of the GI transit conditions in beagle dogs with a telemetric motility capsule. Eur J Pharm Biopharm. 2019;136:221–30.
Walsh PL, Stellabott J, Nofsinger R, Xu W, Levorse D, Galipeau K, et al. Comparing dog and human intestinal fluids: implications on solubility and biopharmaceutical risk assessment. AAPS PharmSciTech. 2017;18(4):1408–16. https://doi.org/10.1208/s12249-016-0611-2.
Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351–80.
He YL, Murby S, Warhurst G, Gifford L, Walker D, Ayrton J, et al. Species differences in size discrimination in the paracellular pathway reflected by oral bioavailability of poly(ethylene glycol) and D-peptides. J Pharm Sci. 1998;87(5):626–33.
Borde AS, Karlsson EM, Andersson K, Björhall K, Lennernäs H, Abrahamsson B. Assessment of enzymatic prodrug stability in human, dog and simulated intestinal fluids. Eur J Pharm Biopharm. 2012;80(3):630–7.
Haller S, Schuler F, Lazic SE, Bachir-Cherif D, Krämer SD, Parrott NJ, et al. Expression profiles of metabolic enzymes and drug transporters in the liver and along the intestine of beagle dogs. Drug Metab Dispos. 2012;40(8):1603–10.
Martinez MN, Mistry B, Lukacova V, Lentz KA, Polli JE, Hoag SW, et al. Exploring canine-human differences in product performance. Part 12. II: use of modeling and simulation to explore the impact of formulation on ciprofloxacin in vivo absorption and dissolution in dogs. AAPS J. 2017;19(3):712–26.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23.
Bowman CM, Benet LZ. Hepatic clearance predictions from in vitro-in vivo extrapolation and the biopharmaceutics drug disposition classification system. Drug Metab Dispos. 2016;44(11):1731–5.
El-Kattan AF, Varma MV, Steyn SJ, Scott DO, Maurer TS, Bergman A. Projecting ADME behavior and drug-drug interactions in early discovery and development: application of the extended clearance classification system. Pharm Res. 2016;33(12):3021–30.
Bowman CM, Benet LZ. An examination of protein binding and protein-facilitated uptake relating to in vitro-in vivo extrapolation. Eur J Pharm Sci. 2018;123:502–4.
Svennebring AM. Investigation of the prerequisites for the optimization of specific plasma protein binding as a strategy for the reduction of first-pass hepatic metabolism. Xenobiotica. 2015;45(4):286–301.
Hung DY, Mellick GD, Whitehead BD, Roberts MS. The effect of protein binding on the hepatic first pass of O-acyl salicylate derivatives in the rat. J Pharm Pharmacol. 1998;50(1):63–9.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–47.
Hosey CM, Chan R, Benet LZ. BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs. AAPS J. 2016;18(1):251–60.
Varma MV, Steyn SJ, Allerton C, El-Kattan AF. Predicting clearance mechanism in drug discovery: extended clearance classification system (ECCS). Pharm Res. 2015;32(12):3785–802.
Adson A, Raub TJ, Burton PS, Barsuhn CL, Hilgers AR, Audus KL, et al. Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. J Pharm Sci. 1994;83(11):1529–36.
Van De Waterbeemd H, Smith DA, Beaumont K, Walker DK. Property-based design: optimization of drug absorption and pharmacokinetics. J Med Chem. 2001;44(9):1313–33.
Matsumura N, Yamaura Y, Katagi J, Ono S, Kim S, Yamashita S, et al. Evaluation of using dogs to predict fraction of oral dose absorbed in humans for poorly water-soluble drugs. J Pharm Sci. 2018;107(9):2489–96.
Flanagan SD, Takahashi LH, Liu X, Benet LZ. Contributions of saturable active secretion, passive transcellular, and paracellular diffusion to the overall transport of furosemide across adenocarcinoma (Caco-2) cells. J Pharm Sci. 2002;91(4):1169–77.
Adson A, Burton PS, Raub TJ, Barsuhn CL, Audus KL, Ho NF. Passive diffusion of weak organic electrolytes across Caco-2 cell monolayers: uncoupling the contributions of hydrodynamic, transcellular, and paracellular barriers. J Pharm Sci. 1995;84(10):1197–204.
Lee K, Thakker DR. Saturable transport of H2-antagonists ranitidine and famotidine across Caco-2 cell monolayers. J Pharm Sci. 1999;88(7):680–7.
Halpin RA, Geer LA, Zhang KE, Marks TM, Dean DC, Jones AN, et al. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos. 2000;28(10):1244–54.
Maaland MG, Guardabassi L, Papich MG. Minocycline pharmacokinetics and pharmacodynamics in dogs: dosage recommendations for treatment of methicillin-resistant Staphylococcus pseudintermedius infections. Vet Dermatol. 2014;25(3):182–e47.
Jung D, Powell JR, Walson P, Perrier D. Effect of dose on phenytoin absorption. Clin Pharmacol Ther. 1980;28(4):479–85.
Luke DR, Foulds G. Disposition of oral azithromycin in humans. Clin Pharmacol Ther. 1997;61(6):641–8.
Van der Heyden S, Vercauteren G, Daminet S, Paepe D, Chiers K, Polis I, et al. Expression of P-glycoprotein in the intestinal epithelium of dogs with lymphoplasmacytic enteritis. J Comp Pathol. 2011;145(2–3):199–206.
Mouly S, Paine MF. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm Res. 2003;20(10):1595–9.
Abraham MH. Human intestinal absorption--neutral molecules and ionic species. J Pharm Sci. 2014;103(7):1956–66.
Sjögren E, Westergren J, Grant I, Hanisch G, Lindfors L, Lennernäs H, et al. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim. Eur J Pharm Sci. 2013;49(4):679–98.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies
Rights and permissions
About this article
Cite this article
Martinez, M.N., El-Kattan, A., Awji, E. et al. Reconciling Human-Canine Differences in Oral Bioavailability: Looking beyond the Biopharmaceutics Classification System. AAPS J 21, 99 (2019). https://doi.org/10.1208/s12248-019-0364-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-019-0364-4