Abstract
Background
Current health care demonstrates an insufficient provision and utilization of physical exercises despite their recommendation as a first-line treatment in clinical guidelines for patients with knee osteoarthritis (OA). Mobile health (m-health) technologies offer new opportunities to guide and monitor home-based exercise programs by using mobile devices and inertial sensors in combination with a digital application (app). This study will evaluate patient benefits resulting from the use of the specific digital health application re.flex for patients with knee OA.
Methods
This monocentric, two-arm, randomized controlled parallel-group trial will evaluate the effectiveness of the app- and sensor-guided exercise program re.flex for patients with moderate-to-severe knee OA. We aim to recruit 200 participants via newspapers, newsletters and information events. Participants will be randomly allocated to the intervention group and the control group in a 1:1 ratio. Participants in the control group will not receive any study intervention or instruction for any change to their previous health care utilization. Despite this, they are allowed to make use of usual care provided by their treating physician. The intervention group comprises a 12-week home training program with three sessions per week in addition to usual care. Exercises will be guided and monitored by use of the training app (re.flex) and two accelerometers that are attached proximally and distally to the affected knee joint. Pre- and postmeasurements will take place at baseline (t0) and after 12 weeks (t1). Primary outcomes will be osteoarthritis-specific pain and physical function measured with the Knee Osteoarthritis Outcome Score (KOOS) subscales Pain and Function in daily living (ADL). Second, further self-reported health outcomes, a performance measurement, app logfiles and safety will be assessed. Intervention effects will be calculated by baseline-adjusted analysis of covariance (ANCOVA) using an intention-to-treat approach. Multiple imputation will be applied.
Discussion
Re.flex can bridge part of the gap between recommendations for strengthening exercises in patients with knee OA and the insufficient actual care situation. This randomized controlled trial is designed to provide conclusions on the effectiveness of the health application re.flex for the population under study and will provide further insight into adherence rates and the safety of its use.
Trial registration
The trial was registered on 20/01/2023 at www.drks.de (ID: DRKS00030932).
Similar content being viewed by others
Background
Osteoarthritis (OA) is the most common degenerative joint disorder in Germany and worldwide [1]. According to the GEDA 2014/2015-EH Interview Survey of the Robert Koch Institute, the 12-month prevalence of OA in adults in Germany is assessed at 17.9% [2]. It is estimated that more than half of those affected suffer from knee OA [3]. Older age, female sex and biomechanical stress induced by overuse or malalignment as well as previous injuries or overweight are potential risk factors for OA [2].
Disease progression is frequently associated with increasing pain and growing limitations in physical functioning and health-related quality of life. Due to its progressive degenerative character, interventions primarily aim for a reduction in disease symptoms and improvements in physical functioning. They further intend to foster disease self-management and patient education. Medical guidelines worldwide recommend physical exercises and patient education as nonpharmacological core treatments for patients with knee OA [4, 5]. However, there is an insufficient provision and utilization of these first-line treatments in current health care. A recent meta-analysis of 15 studies evaluating the quality of OA care in the community concluded that less than 40% of eligible OA patients received recommendations to exercise [6]. Furthermore, only 35% of the German people with OA reported that they engage in strength training at least twice a week [7]. Data from a German health insurance company show that more than 60% of the insurance holders aged 60 years and above and diagnosed with OA received medication compared with only 41% receiving a prescription for physiotherapy [8]. The latter does not necessarily include exercise instructions [9].
From a mechanical perspective, strengthening exercises aim to stabilize the affected joint and decrease abnormal joint loads by improving muscle strength and neuromuscular control [10, 11]. To date, many studies on exercise therapy in knee OA have shown increased strength in the short and mid-term [12, 13]. In this regard, adherence to exercise seems to be crucial, as exercise interventions are categorized as lifestyle interventions. Initial instructions are given, after which the exercises should be continued in a self-dependent manner [14]. An ongoing need for the development of highly stimulating exercise programs designed for long-term adherence can therefore be postulated [13]. In this regard, home-based exercise programs seem to be of particular relevance, as they can be conducted independently [10]. Digital health applications have great potential to support patients in performing exercises correctly and safely. The outstanding advantages of digital health applications are related to their extensive availability, allowing users to be instructed in exercises independent of time and location. They further appeal to a wide range of possible users [15,16,17] and have the possibility of closer monitoring, such as an objective method of measuring adherence to exercise by use of additional motion sensors [18]. In a randomized controlled trial, Bennell and colleagues [19] compared home exercise prescribed by therapists’ usual methods with home exercise prescribed using a web-based exercise programming system for people with musculoskeletal conditions. Conclusively, web-based home exercise resulted in improved adherence and self-confidence in the ability to perform exercises independently.
The digital transformation in health care is advancing rapidly. One example of this ongoing process is the Digital Care Law in Germany, which came into force in 2019 [20]. This law aims for better care using digitalization and innovation. Among others, it allows patients to gain access to specific health apps that are listed in a register for disease-specific digital health applications (DiGA). Costs for DiGAs are reimbursed from the statutory health insurance companies. However, evidence of a positive care effect, such as patient benefit, is a prerequisite for the final inclusion of a DiGA into the reimbursable DiGA register [21]. To create incentives using digital interventions, such or similar reimbursement models are needed from a clinician’s perspective [22].
This randomized controlled trial will investigate the patient benefits associated with the preliminary listed DiGA re.flex that is used to instruct and guide exercises for patients with knee OA.
Objectives
The main objective of this study is to investigate the effectiveness of a 12-week sensor-assisted and app-supported exercise intervention in addition to usual care (intervention group) in comparison to a control group with usual care only (control group).
The two primary endpoints are the comparison of baseline-adjusted scores between the intervention and control group regarding osteoarthritis-specific pain (subscale Pain of the Knee Osteoarthritis Outcome Score, KOOS) and physical function (subscale Function in daily living (ADL) of the Knee Osteoarthritis Outcome Score, KOOS) directly after the 12-week intervention phase. Other study endpoints include further self-reported health outcomes, a performance measurement, app logfiles and safety aspects (Table 2).
Methods
Study design
The study is designed as a monocentric pragmatic two-armed randomized controlled superiority trial and will be conducted in an academic hospital with an outpatient clinic for Sports Medicine located in Tübingen, Germany. Subjects are randomly allocated to the intervention group and control group in a 1:1 ratio with n = 100 in each group.
Pre- and postmeasurements will take place at baseline (t0) and after 12 weeks (t1). The study was prospectively registered in the German Clinical Trial Register (DRKS00030932) on 20/01/2023 and will be reported following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist [23] (Supplement 1).
Eligibility criteria
Eligible patients are those of any sex ≥ 18 years suffering from knee OA (International Classification of Disease, ICD codes M17.0–17.5 and 17.9). The knee must be the primary location of OA symptoms. Knee OA is first diagnosed via self-reported previous OA diagnosis by a physician according to the wording of the GEDA questionnaire [2]. Patients are further asked to rate their OA severity via the Subscale Pain of the KOOS [24, 25]. Only patients with at least moderate self-reported symptoms at the time of screening are eligible for the study (KOOS pain ≤ 60 points, where 100 points indicate no complaints at all). To address that criterion, the KOOS pain subscale will be administered by phone and based on possible symptoms that typically occurred during the last week. OA diagnosis is verified in the context of the physical examination at t0 by a physician (orthopaedist). If available, the diagnosis is confirmed using X-ray or MRI images provided by the patient. Further inclusion and exclusion criteria are outlined in Table 1.
Intervention
Control group
Participants in the control group will not receive any study intervention or instruction for any change to their previous health care utilization. They are allowed to make use of usual health care provided by their treating physician, if applicable.
Usual care is defined as any kind of prescribed pharmacological or physical intervention a patient with knee OA usually receives when consulting a medical doctor because of knee OA. These may include physical therapies such as regular physiotherapy, manual therapy, electrotherapy, orthotic devices and medical prescriptions for pharmacological agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) [28]. These reflect the relevant treatment options according to the current national guidelines in Germany [4].
App-guided exercise intervention for knee osteoarthritis (intervention group)
The exercise intervention comprises a 12-week app-guided home training program that was specifically designed for patients with knee osteoarthritis. Exercises are guided by use of the training app and two accelerometers (re.flex, © 2019, KINETO TECH REHAB SRL) that are attached proximally and distally to the affected knee joint (Fig. 1).
The primary focus of the intervention is to strengthen knee extensors, knee flexors and hip abductors. Further exercises aim for joint mobilization, muscle flexibility and balance training.
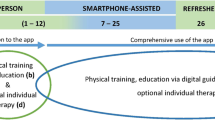
The first two weeks focuses on familiarization with different kinds of exercises and exercise loads. In this regard, patients should be enabled to adapt exercise intensity self-determinately according to perceived strain and pain. Therefore, patients can choose between two different intensity grades. They further have to comment about their strain and pain levels after each set of exercises (see outcome measures). The following four weeks are designed to increase strength endurance, enhance the range of motion of the lower extremities and improve balance ability. In this phase, strengthening exercises should be performed with 2 sets of 25 repetitions (Fig. 2).
The last six weeks of the intervention mainly focus on muscle hypertrophy with higher intensities and lower repetition numbers. Strength training should be performed with 3 sets of 15 repetitions in this phase. Moreover, balance and range of motion should be further improved (Fig. 2). From week 5 onwards, the training program can partly be modified by the users by choosing their preferred exercises for some of the strength, mobility, flexibility or balance exercises out of the exercise pool that they became familiar with during previous sessions. Alternative exercises relate to the same musculature and task, yet they may differ regarding the exercise position or an open versus closed kinetic chain.
The use of the app and sensors will be introduced in the context of the baseline examination on site by the pretrained study staff, and patients will further receive a manual for using the software and hardware.
The app acts as a virtual training partner, providing exercise descriptions and videos (Fig. 1), setting the number of repetitions and sets, predefining joint angles and the related range of motion for the exercises and defining the movement velocity of the exercises.
Each of the 12 weeks foresees 3 exercise sessions with a respective duration of 25–30 min each, which can be performed independently of location and time. Different types and variations of exercise (i.e., the use of long or short lever arms or different starting positions: supine, seated, standing) as well as elastic exercise bands are used to allow progression of training loads. All one-sided exercises are conducted alternately with each leg to avoid muscular imbalances. Furthermore, subjects will be instructed prior to the start of the intervention that strength exercises are effective when the final two to three repetitions are perceived as strenuous to very strenuous [7, 8] on the RPE (rating of perceived exertion) scale 0–10. If at the end of the exercise it is realized that the exercise was too easy or too difficult, the exercise intensity can be adjusted the next time the exercise is performed by choosing a more difficult or easier variation of the exercise.
The app further reminds the user to conduct upcoming training sessions via push notifications. All training sessions, including the number of repetitions actually performed compared to the given number of repetitions of an exercise, are tracked by the two accelerometers and thus can help to evaluate training adherence in the future. Throughout the intervention phase, users can contact the provider via the app messenger. The provider is responsible for clarification of technical issues. Participants are instructed to interrupt the training program in case of any suspicious symptoms, fatigue or excessive pain during the exercise intervention with the re.flex system. They are asked to inform the study personnel about any adverse event to allow judgement on how to proceed. This decision is up to the study physician as well as the study personnel (sports scientist/physiotherapist) and will refer to the options of modification of the training regime or its temporary or complete discontinuation.
In correspondence to the control group, participants of the intervention group are allowed to make use of usual health care provided by their treating physician, if applicable.
Outcome measures
The following Table 2 gives an overview of all outcome measures, their study instruments with references and their time points of assessment. Details on each measure are provided in the subsequent sections. Patient-reported outcome measures (PROMs) will be assessed using an online questionnaire (Questback GmbH, Köln, Germany) during the examinations at the study site (t0, t1) and at home (only applicable for concomitant care at 4 and 8 weeks). The physical performance measure will be assessed on-site at t0 and t1. Sociodemographic data, clinical status (e.g., relevant previous injuries or surgeries, comorbid conditions) [29], outcome expectations [30], previous experiences with strengthening exercises and physical activity as well as technical affinity [31] and fear of movement [32] are assessed in addition to the primary and secondary outcomes. The participants will receive an e-mail prior to the data collection, reminding them of the upcoming data collection. All used survey instruments are license-free or, in the case of the Veterans RAND 12-Item Health Survey (VR-12), permission was obtained from the authors.
The selection of outcome measures aligns with the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) core domain set for trials of people with hip and knee osteoarthritis [40]. The selection also aligns with the Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group (ICHOM) on Standard Outcome Measures for hip and knee OA released in 2016 [29]. The mandatory set of outcome measures is supplemented by further relevant outcome measures in the context of exercise therapy and m-Health.
Primary outcomes
The KOOS is a widely accepted and comprehensive outcome measure for several domains, including pain and physical function. It was developed as a nonproprietary comprehensive extension to the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and has been proven to be valid, reliable, and responsive to OA outcomes [25, 29]. The test–retest reliability of the German version was in the range of rs = 0.65–0.78, which corresponds to a sufficiently high reliability for all subscales. The validity was determined by comparing the subscales with the patient’s self-assessment of health status and the results of the SF-12 (quality of life questionnaire). A sufficiently high convergence validity was shown [24]. The Knee Injury and Osteoarthritis Outcome Score (KOOS) [24, 25] was developed to assess the patient’s opinion about their knee and associated problems. The KOOS evaluates both short-term and long-term consequences of knee injury and consequences of primary OA. It contains 42 items in five separately scored subscales: KOOS Pain, KOOS Symptoms, Function in daily living (KOOS ADL), Function in Sport and Recreation (KOOS Sport/Rec), and knee-related Quality of Life (KOOS QoL). All scores will be included; however, the KOOS Pain score and the KOOS ADL score are the primary outcomes of the study.
Secondary and other outcomes
Patient-reported outcome measures (PROMs)
-
(1)
Further OA-specific complaints
Further subscales from the KOOS, such as the subscales Symptoms, Function in Sport and Recreation (Sport/Rec) and knee-related Quality of Life (QoL), will be analysed to consider a wide range of OA-specific complaints.
-
(2)
Patient global assessment
The patient’s global assessment of knee osteoarthritis will be used as a one-item scale “Considering all the ways your osteoarthritis in your knee affects you, how are you doing today?” using a 5-point Likert scale (1-very good: asymptomatic and no limitation of normal activities; 2-good: mild symptoms and no limitation of normal activities; 3-fair: moderate symptoms and limitation of some normal activities; 4-poor: severe symptoms and inability to carry out most normal activities; 5-very poor: very severe symptoms that are intolerable and inability to carry out all normal activities). The description for each answer category refers to Schnitzer and colleagues and will be translated into the German language [33].
-
(3)
Health-related quality of life
The Veterans RAND 12-Item Health Survey (VR-12) [34] is a generic questionnaire to assess the patient’s opinion about their health-related quality of life. The VR-12 allows the calculation of a mental as well as a physical component scale and includes a 1-item scale for general health.
-
(4)
Subjective assessment of overall change, change in pain and function
The following transition questions will be used to assess the subjective change in health status at t1: “Please imagine how your health status was 3 months ago. How do you feel about your osteoarthritis in the index knee joint today compared to 3 months ago? Please mark the answers that are applicable: (1) in general; (2) in the area of pain; (3) in the area of walking.” The questions are each answered with a 5-point Likert scale with the response options “much better”, “somewhat better”, “unchanged”, “somewhat worse” and “much worse”. The questions and answers refer to Angst and colleagues and were translated into German by the author [35].
Objective measures
The 30-s chair stand test [36] aims to test leg strength as well as leg strength endurance. The participant is seated in the middle of a chair with the hands and arms crossed in front of the upper body. Feet are completely positioned on the floor, and the back is straight. Out of this initial position, the participant is asked to stand up until an upright position and to sit again as often as possible within a 30-s time window. The results can be related to sex- and age-matched norm values.
Concomitant care
Utilization of previous care and treatment will be assessed at t0 with a retrospective time window of 12 months. Utilization of concomitant care (yes vs. no entry) during the study period will be assessed 4 and 8 weeks after baseline and at t1 (twelve weeks after baseline) with a retrospective time window of 4 weeks. Assessments will include the type of care utilization (i.e., physical therapist, general practitioner, dietician, etc.) and the type of received treatment referring to different kinds of information/advice, self-managed care, nonsurgical care, clinical care and surgery [29]. Within the categories self-managed care/clinical care, medication intake (regularly, sporadic, no), the type of medication taken, and its dose and frequency of intake will explicitly be asked for.
Perceived human-digital interaction, patient satisfaction
The perceived usability of the digital health application will be assessed with the m-Health App Usability Questionnaire (MAUQ) [37] in addition to 1 item from Harder et al. [38] (Item B1). The MAUQ is not available in a validated German language version and is used in a self-translated version. The assessment of this questionnaire takes place after the exercise intervention period.
The modified version of the 8-item scale ZUF-8 will be used to assess patient satisfaction with the received care [39]. The modifications relate to changes according to the kind of care (stand-alone app here versus hospital setting in the original version).
Patient perspective on the satisfaction with the results of their treatment in general will be asked with the one-item question “How satisfied are you with the results of your treatment?” using a 5-point Likert scale (very satisfied; satisfied; neither satisfied nor dissatisfied; unsatisfied; very unsatisfied) [29].
Logfiles
Logfiles of the re.flex digital application for the evaluation of adherence to exercise, rating of perceived exertion during exercising and perceived pain before, during and after exercising will be read out for each training session separately.
-
(1)
Adherence to exercise
Overall adherence will be quantified using the percentage of conducted exercise sessions relative to the overall number of prescribed exercise sessions, irrespective of compliance with the prescribed exercise dosage. In addition, logfiles of the app provide detailed information on the number of valid repetitions and sets of each exercise within a given training session as well as overall data for each training session during the 12-week intervention phase for the sensor-equipped leg. Training adherence is therefore further quantified by calculating the percentage of all valid repetitions conducted with the affected leg during the training related to the number of requested repetitions. The overall exercise repetition adherence is the mean value of the adherence of all training sessions of the 12-week program considering the calculation procedure as mentioned before. Further data include the time in action as well as the total training time (including calibration, instruction, training of the other leg, etc.).
-
(2)
Rating of perceived exertion (RPE) during exercising
Participants are asked to rate their exertion using the entry field of the app after each set of exercises as well as overall exertion at the end of the training session. RPE is measured using a numerical rating scale (NRS) with 0 indicating no exertion at all and 10 indicating the maximal conceivable exertion. Values are read out for each set of an exercise separately. Average values are calculated.
-
(3)
Perceived pain before, during and after exercising
Pain ratings are requested before and after each training as well as after each exercise set. Values are read out for each exercise separately. Patients are asked to rate their pain using the entry field of the app. Perceived pain is measured using the Faces Pain Scale (FPS-R) [41]. Face emojis are scored 0 (no pain), 2 (little pain), 4 (moderate pain), 6 (much pain), 8 (very much pain) or 10 (highest imaginable pain). In case of an entry with a pain index of more than 8, the user gets a recommendation to pause training and to contact the physician in charge.
The In-app pain report is not aimed at replacing the comprehensive KOOS. Rather, it is intended to be used to monitor pain levels throughout the course of the exercise intervention.
Safety aspects
Exercise-related pain and adverse events will be assessed retrospectively at t1, including frequency, duration, intensity, and potential causes. Exercise-related pain will only be assessed in participants in the intervention group, and adverse events (AEs) will be assessed in both study arms.
Participants are informed at t0 to contact the study personal in case of AEs during the study period. Minor AEs must be reported to the responsible study personnel within one week (postal letter, email, telephone, in-app support chat). Adverse events causing the need for referral to a physician or hospital have to be reported to the responsible study personnel immediately. Judgement on whether the AE is related to the intervention is carried out by an orthopaedist or physician for internal medicine of the Dept. of Sports Medicine. In case of an unclear connection between an AE and the intervention or in case of a serious AE, representatives of the study sponsor Sporlastic and other study independent physicians of the University Hospital will report the situation to a safety board. This safety board will then decide on the continuation or premature termination of the trial for safety reasons.
Participant timeline and recruitment
The first patient was recruited in January 2023. Continued inclusion of patients is planned until the end of April 2023. Assessments at baseline (t0) and immediately after the 12-week intervention/control phase (t1) will be conducted on-site at the University Hospital, Tübingen, Germany, and data will be collected as outlined in Fig. 3 and Table 2. Assessors for the performance outcome measure are thoroughly instructed by the study personnel who are responsible for the use of the measurement instrument as well as for all other assessments, including the randomization process prior to the start of the study. Prior to the independent execution of the tests, all assessors performed up to three supervised assessments until these were carried out correctly. Additionally, an online questionnaire asking for concomitant care will be sent at weeks 4 and 8. All participants are expected to have completed the study by the end of July 2023.
A detailed timeline for the individual patients can be seen in the study flow chart in Fig. 3.
Potential study participants will be recruited via newspaper, newsletter for employees of the University Hospital and the University and people who were interested in participating in a previous study (DRKS00023269) but could not be included because of bicondylar symptoms (eligibility criteria have been changed for this trial).
Recruiting will take place in a two-step procedure. The first contact will be held by telephone or email. The purpose of this contact is to inform the interested person about the content and aims of the study as well as its timeline. The first screening for eligibility by querying the inclusion and exclusion criteria is also part of this contact. In this regard, the Physical Activity Readiness-Questionnaire PAR-Q is used to screen for exercise suitability. If one or more questions related to restrictions other than musculoskeletal are answered with “yes”, the interested person must provide a confirmation for cardio-pulmonal exercise suitability from his personal doctor in case of further interest in study participation. This step takes place before study inclusion and must be done on the patient’s initiative.
In the context of the second step, the eligible patient is invited to a face-to-face meeting. This meeting includes comprehensive oral and written study information and the option for the patient to ask and respond to open study-related questions. After providing written consent, the patient is included on a provisional basis and referred to the study physician (orthopaedist) for medical examination (anamnesis and physical examination). The patient is finally included in case of alignment to inclusion criteria and absence of exclusion criteria. Otherwise, the subject is definitely excluded before randomization takes place.
The examination is followed by the outcome assessments. Finally, patients will be randomized to the intervention or control group. This first baseline examination will take approximately two hours. The first examination (t0) is followed by the 12-week intervention or control phase. Follow-up measures (t1) will be conducted directly after the 12-week intervention or control phase. The complete study duration for each participant will be approximately 14 weeks, including two assessments as well as the 12-week intervention or control phase.
Sample size
The two primary endpoints of the study are the KOOS subscales of pain and ADL. The results of a pilot study comparing the KOOS pain subscale and KOOS ADL subscale of 29 patients in the control group (usual care) with 15 patients in the intervention group (re.flex) using baseline adjusted analysis of covariance revealed effect sizes of 1.16 (Pain subscale) and 1.03 (ADL subscale). A recent meta-analysis on exercise interventions in patients with knee OA reported a standardized mean difference of 0.5 (95% CI 0.37 to 0.63) for pain reduction immediately posttreatment in comparison to usual care or minimal treatment [42]. Additionally, minimal clinically important differences (MCIDs) were reported between 5.5 and 8.7 points on the WOMAC pain subscale (score 0–100) for nonsurgical treatment strategies in patients with knee OA [35, 43]. These correspond to standardized mean differences between 0.59 and 0.94. Based on the preceding, rather heterogeneous results with effect sizes between 0.5 and 1.2 from the results of the pilot study and findings and recommendations of other sources, the planned study is powered to demonstrate a MCID of 5 points (0–100) on the KOOS Pain Subscale between the intervention and control group with a standard deviation of 10. This leads to an effect size of 0.5 and to a sample size of evaluable participants of 2*78 = 156. For adjustment of baseline, aetiology, medication and laterality, 4 additional degrees of freedom are spent, and the sample size is increased to 160. Considering a drop-out rate of ~ 20%, 200 patients will be recruited to achieve a power of 80% with a type 1 error of 0.025 (two-sided Bonferroni correction for two confirmatory outcomes) by baseline adjusted comparison of outcome values at t1 between study arms (analysis of covariance, ANCOVA).
Randomization
Before the study start, randomization lists will be created for each of the eight combinations of the strata aetiology (primary, secondary), medication (regularly/no or sporadic) and laterality of the disease (one-sided, two-sided) using computer-generated random numbers (0;1) with varying block lengths and 50 subjects per stratum combination. The randomization list will be transferred to the data management system SecuTrial (Interactive Systems Berlin). Online randomization will be performed after confirmation that the respective subject fulfils all selection criteria, after baseline assessments take place and after entry of the stratification criteria. Randomization will be performed on site using the randomization tool SecuTrial and will be conducted in the order of patient appearance by the assessors.
Participants will not be randomized in case of exclusion before completion of the examination and tests at t0.
Blinding
The study intervention is obvious, and therefore, an adequate comparable placebo intervention is not possible. As such, this trial is nonblinded for participants. Blinding of health care providers is not applicable, as the intervention of interest is a stand-alone app without human interaction. Baseline assessments will take place before randomization, and data collectors for the performance test will be blinded to the group allocation of the participants for follow-up assessment. All other outcomes are self-reported, and blinding is not possible, as they include outcomes that are only assessed in the intervention group (e.g., logfiles, patient satisfaction with the app); thus, group allocation is obvious. The creation of the computer-generated randomization list using the software nQuery will be done by an employee of the IKEAB who is not involved in the conduction and assessment of the study. Statisticians will be blinded for the analyses of the primary endpoints and all other health outcome measures. For further analyses, statisticians will be unblinded, as treatment allocation is obvious (i.e., perceived human-digital interaction, logfiles, etc.).
Statistical methods
The two primary endpoints of the statistical analyses are the KOOS subscales pain and KOOS ADL. They will be analysed at t1 immediately after the termination of the intervention using a baseline adjusted analysis of covariance (ANCOVA) with the primary factor “study arm”. The level of significance will be 0.025 (two-sided Bonferroni correction for two confirmatory outcomes). We hypothesize that the exercise intervention will be superior to the control. Additionally, the stratification factors aetiology (primary, secondary), medication (regularly/no or sporadic) and laterality of the disease (one-sided, two-sided) will be coded by binary variables. The model equation is:
Y: KOOS (Pain resp. ADL) at t1, BL: baseline KOOS (Pain resp. ADL), Arm: study arm coded as 0 (control) and 1 (exercise intervention), ε random error (normally distributed, equal variance, independent). Reference categories “primary”, “no or sporadic medication”, and “one-sided disease”, H0: β2 = 0, H1: β2 ≠ 0 (two-sided test, scientific hypothesis: superiority of exercise intervention, i.e., β2 < 0).
Continuous secondary endpoints will be analysed using the same statistical methods (analysis of covariance (ANCOVA) if baseline values are available; otherwise, analysis of variance else). For the main results, two-sided 95% confidence limits will be given in addition to significance tests.
The success of randomization will be assessed using baseline comparisons between both study arms. The primary analysis population will be the intent-to-treat population. This population includes all patients who contribute at least baseline values of the primary outcomes. Multiple imputation will be applied to subjects who drop out or do not contribute measurements of the primary outcome for other reasons. Five hundred imputation samples will be drawn. Baseline measurements will be included as predictors (c.f. definition of intent-to-treat population). The “jump to reference” method will be used for the primary analysis, i.e., patients who drop out will be assigned to the control arm in the imputation model but not in the analysis model. Sensitivity analyses will be performed with “complete case analysis”, “baseline observation carried forward” (single imputation) and with inclusion of the correct study arm in the imputation model (multiple imputation). A per protocol analysis will be conducted with all participants of the intervention group complying with the study criteria and still using the app until the last two weeks of the intervention phase with an overall adherence rate of 80% of the scheduled sessions. The same procedure will be performed for all other secondary health outcome measures for which baseline values are complete.
Exploratory subgroup analyses will be performed for the stratification factors and patient age (18–40 years, 40–55 years, 55–65 years, older than 65 years). Additionally, in the case of frequent or differential occurrence of concomitant care (medication, physical therapy – active treatment) between the study groups, an exploratory subgroup analysis will be performed. P values for interaction of study arm with stratification factors and patient age as well as p values of study arms within strata will be reported but should not be interpreted as confirmatory.
An additional analysis is planned for subjective ratings of overall change (general, pain, function). Response scales will be first dichotomized into improved (somewhat/much better) and not improved (unchanged or worse). Between-group comparisons will be expressed as relative risks of improvement [44]. Exploratory, prognostic factors, including baseline pain, age, BMI, sex, technical affinity and fear of movement, will be analysed using multiple regression models (linear regression) to identify potential responders to the training. Binary outcomes will be analysed using similar logistic regression models.
Data management
Data from all participants with informed written consent will be pseudonymized. On-site data will be captured with paper and pencil case report forms (CRFs) and using an online questionnaire (Questback GmbH, Köln, Germany) for PROMS. Log-Data of app usage will be captured by the software and transferred to the study team (*.csv-file). Paper and pencil CRFs will be double entered into an electronic data sheet (Excel). Doubly entry will be checked for errors. The data bank will be closed after the last patient out and after all queries are processed. Study data are then exported via *.csv-File to the statistical programs in use.
Data monitoring
There is no external data monitoring committee for this study.
Discussion
M-health interventions such as the sensor-guided digital-based exercise intervention re.flex can be used independently from time and location and allow most patients to gain access to this kind of exercise guidance. Re.flex was specifically developed to support home training in patients with knee OA. If effective, it can bridge part of the gap between recommendations for strengthening exercises in patients with knee OA and the insufficient actual care situation. However, to be classified as a digital health application reimbursed by German health insurance companies, the intervention must prove patient benefit. There are several ways to define appropriate controls for digital therapeutic clinical trials, e.g., using a sham control intervention such as a low-functional digital health tool lacking the elements to be reasonably effective [45]. The selection of the control group in this trial agreed with the responsible authority (Federal Institute for Drugs and Medical Devices in Germany, BfArM).
Limitations
The following limitations must be addressed for the outlined study. All participants are allowed to make use of usual health care provided by their treating physician during the study period. This concerns the intervention and control group, and cannot be prohibited, as all persons with German health insurance have a right to receive physician-recommended usual care treatments. Exclusive participation in re.flex (intervention) or doing nothing (control) cannot be ensured. To address this issue, all concomitant care will be queried retrospectively for one year and every 4 weeks until the end of the study.
Considering the intervention duration of 12 weeks, only short-term effects can be assessed. Long-term effects will not be determined.
Conclusion
This randomized controlled trial is designed to provide conclusions on the effectiveness of re.flex for the population under study. Patient benefit is primarily related to OA-specific pain reduction and improvement of physical function. Superiority of re.flex versus control for pain and function is a prerequisite for the permanent listing of the app into the DiGA register.
In addition, this study will add important knowledge to the scientific community on the short-term effectiveness of exercise-related digital health interventions on health outcomes in general, and it will further provide evidence on its usability, patient acceptance and safety. Regarding this, sensor-based technology allows objective measures of exercise performance and may therefore help to control and analyse exercise training at home.
Availability of data and materials
Data and material will be made available upon reasonable request after the conclusion of the study.
Abbreviations
- ADL:
-
Function in daily living
- AE:
-
Adverse event
- ANCOVA:
-
Analysis of covariance
- App:
-
Application
- CRF:
-
Case report form
- DiGA:
-
Digital health application – Digitale Gesundheitsanwendung
- DRKS:
-
German clinical trials register - Deutsches Register Klinischer Studien
- ETS:
-
Expectation for Treatment Scale
- FPS-R:
-
Faces Pain Scale
- ICD:
-
International Classification of Disease
- ICHOM:
-
International Consortium for Health Outcomes Measurement
- IKEAB:
-
Institute for Clinical Epidemiology and Applied Biometrics
- KOOS:
-
Knee Osteoarthritis Outcome Score
- MAUQ:
-
M-Health App Usability Questionnaire
- MCID:
-
Minimal clinically important difference
- m-health:
-
Mobile health
- NRS:
-
Numerical rating scale
- NSAID:
-
Nonsteroidal anti-inflammatory drugs
- OA:
-
Osteoarthritis
- OARSI:
-
Osteoarthritis Research Society International
- OMERACT:
-
Outcome Measures in Rheumatology
- PAR-Q:
-
Physical Activity Readiness Questionnaire
- PROM:
-
Patient-reported outcome measure
- QoL:
-
Quality of life
- RPE:
-
Rating of perceived exertion
- Sport/Rec:
-
Sport and recreation
- t0:
-
Timepoint 0, collection point at baseline
- t1:
-
Timepoint 1, collection point after 12-week study phase
- TA-EG:
-
Questionnaire to assess technical affinity regarding electronic devices
- TSK-GV:
-
Tampa Scale for Kinesiophobia, German Version
- VR-12:
-
Veterans Rand 12: 12-item questionnaire assessing Health related quality of life
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
- ZUF-8:
-
Patient satisfaction questionnaire
References
Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185–99.
Fuchs J, Kuhnert R, Scheidt-Nave C. 12-Monats-Prävalenz von Arthrose in Deutschland. Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung; 2017.
Fuchs J, Rabenberg M, Scheidt-Nave C. Prävalenz ausgewählter muskuloskelettaler Erkrankungen. Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung; 2013.
Stöve J. S2k-Leitlinie Gonarthrose. AWMF online. 2018;AMWF-Leitlinien-Register Nr 033/004.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89.
Hagen KB, Smedslund G, Østerås N, Jamtvedt G. Quality of community-based osteoarthritis care: a systematic review and meta-analysis. Arthritis Care Res. 2016;68(10):1443–52.
Sudeck G, Geidl W, Abu-Omar K, Finger JD, Krauß I, Pfeifer K. Do adults with non-communicable diseases meet the German physical activity recommendations. Ger J Exerc Sport Res. 2021;51:183–93.
Postler A, Ramos AL, Goronzy J, Gunther KP, Lange T, Schmitt J, et al. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: an analysis based on health insurance claims data. Clin Interv Aging. 2018;13:2339–49.
Zadro J, O’Keeffe M, Maher C. Do physical therapists follow evidence-based guidelines when managing musculoskeletal conditions? Systematic review. BMJ Open. 2019;9(10):e032329.
Krauß I. Sport- und Bewegungstherapie bei Gon- und Coxarthrose. Deutsche Zeitrschrift für Sportmedizin. 2016;67(11):276–81.
Zeng C-Y, Zhang Z-R, Tang Z-M, Hua F-Z. Benefits and mechanisms of exercise training for knee osteoarthritis. Front Physiol. 2021;12:2267.
Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–7.
Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, et al. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31(5):596–611.
Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, Conaghan PG, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–35.
Bossen D, Veenhof C, Dekker J, de BD. The usability and preliminary effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis. BMC Med Inform Decis Mak. 2013;13(1):61.
Bossen D, Veenhof C, Van Beek KE, Spreeuwenberg PM, Dekker J, de Bakker DH. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: randomized controlled trial. J Med Internet Res. 2013;15(11):e257.
Durst J, Roesel I, Sudeck G, Sassenberg K, Krauss I. Comparison of human versus digital instructions for exercise in patients with hip osteoarthritis: results of a non-inferiority randomized cross-over trial. Osteoarthritis Cartilage. 2020;28:S463.
Argent R, Daly A, Caulfield B. Patient involvement with home-based exercise programs: can connected health interventions influence adherence? JMIR Mhealth Uhealth. 2018;6(3):e8518.
Bennell KL, Marshall CJ, Dobson F, Kasza J, Lonsdale C, Hinman RS. Does a web-based exercise programming system improve home exercise adherence for people with musculoskeletal conditions?: a randomized controlled trial. Am J Phys Med Rehabil. 2019;98(10):850–8.
Bundesministerium für Gesundheit. Ärzte sollen Apps verschreiben können. 2020 [updated 22.04.2020]. Available from: https://www.bundesgesundheitsministerium.de/digitale-versorgung-gesetz.html. Accessed 28 Feb 2023.
BfArM. Das Fast Track Verfahren für digitale Gesundheitsanwendungen (DiGA) nach § 139e SGB V. 2020 [updated 23.10.2020]. Available from: https://www.bfarm.de/SharedDocs/Downloads/DE/Service/Beratungsverfahren/DiGA-Leitfaden.pdf;jsessionid=4F25C61118FEF3A4E2930E41479AFDA4.1_cid319?__blob=publicationFile&v=11. Accessed 28 Feb 2023.
Shah N, Costello K, Mehta A, Kumar D. Applications of digital health technologies in knee osteoarthritis: narrative review. JMIR Rehabilit Assistive Technol. 2022;9(2):e33489.
Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Kessler S, Lang S, Puhl W, Stöve J. Der Knee Injury and Osteoarthritis Outcome Score: Ein Funktionsfragebogen zur Outcome-Messung in der Knieendoprothetik. Z Orthop Grenzgeb. 2003;141(3):277–82.
Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1(1):1–8.
Canadian Society for Exercise Physiology (CSEP). Physical Activity Readiness Questionnaire - PAR-Q. 2012. Available from: http://westpointgrey.org/wp-content/uploads/2014/02/PAR-QXXForm.pdf. [Accessed 10 01 2023].
Deutsche Gesellschaft für Sportmedizin und Prävention (DGSP). PAR-Q Fragebogen (deutsche Fassung). 2012. Available from: https://daten2.verwaltungsportal.de/dateien/seitengenerator/leitlinie_vorsorgeuntersuchung_4.10.2007-anlage-1.pdf. [Accessed 10 01 2023].
Lange T, Luque Ramos A, Albrecht K, Gunther KP, Jacobs H, Schmitt J, et al. Prescription frequency of physical therapy and analgesics before total hip and knee arthroplasy : an epidemiological analysis of routine health care data from Germany. Orthopade. 2018;47(12):1018–26.
Rolfson O, Wissig S, van Maasakkers L, Stowell C, Ackerman I, Ayers D, et al. Defining an international standard set of outcome measures for patients with hip or knee osteoarthritis: consensus of the international consortium for health outcomes measurement hip and knee osteoarthritis working group. Arthritis Care Res (Hoboken). 2016;68(11):1631–9.
Barth J, Kern A, Luthi S, Witt CM. Assessment of patients’ expectations: development and validation of the Expectation for Treatment Scale (ETS). BMJ Open. 2019;9(6):e026712.
Karrer K, Glaser C, Clemens C, Bruder C. Technikaffinität erfassen–der Fragebogen TA-EG. Der Mensch im Mittelpunkt Technischer Systeme. 2009;8:196–201.
Rusu AC, Kreddig N, Hallner D, Hülsebusch J, Hasenbring MI. Fear of movement/(Re) injury in low back pain: confirmatory validation of a German version of the Tampa Scale for Kinesiophobia. BMC Musculoskelet Disord. 2014;15(1):1–9.
Schnitzer TJ, Easton R, Pang S, Levinson DJ, Pixton G, Viktrup L, et al. Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. JAMA. 2019;322(1):37–48.
Iqbal S, Rogers W, Selim A. The Veterans RAND 12 Item Health Survey (VR-12): What It Is and How It Is Used. Bedford, Mass, Veterans Administration Medical Center. Center for Health Quality, Outcomes and Economic Research, and Boston University School of Public Health, Center for the Assessment of Pharmaceutical Practices. 2007.
Angst F, Benz T, Lehmann S, Aeschlimann A, Angst J. Multidimensional minimal clinically important differences in knee osteoarthritis after comprehensive rehabilitation: a prospective evaluation from the Bad Zurzach Osteoarthritis Study. RMD Open. 2018;4(2):e000685-e.
Centers for Disease Control and Prevention. Assessment 30-Second Chair Stand. 2017. Available from: https://www.cdc.gov/steadi/pdf/STEADI-Assessment-30Sec-508.pdf. [Accessed 10 01 2023].
Zhou L, Bao J, Setiawan IMA, Saptono A, Parmanto B. The mHealth APP usability questionnaire (MAUQ): development and validation study. JMIR Mhealth Uhealth. 2019;7(4):e11500.
Harder H, Holroyd P, Burkinshaw L, Watten P, Zammit C, Harris PR, et al. A user-centred approach to developing bWell, a mobile app for arm and shoulder exercises after breast cancer treatment. J Cancer Surviv. 2017;11(6):732–42.
Schmidt J, Wittmann WW, editors. Fragebogen zur Messung der Patientenzufriedenheit. Diagnostische Verfahren in der Psychotherapie. Göttingen: Hogrefe; 2002.
Smith TO, Hawker GA, Hunter DJ, March LM, Boers M, Shea BJ, et al. The OMERACT-OARSI core domain set for measurement in clinical trials of hip and/or knee osteoarthritis. J Rheumatol. 2019;46(8):981–9.
International Association for the Study of Pain. Faces Pain Scale. 2001. Available from: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1519&navItemNumber=577. [Accessed 10 01 2023].
Verhagen AP, Ferreira M, Reijneveld-van de Vendel EAE, Teirlinck CH, Runhaar J, van Middelkoop M, et al. Do we need another trial on exercise in patients with knee osteoarthritis: No new trials on exercise in knee OA. Osteoarthritis Cartilage. 2019;27(9):1266–9.
Angst F, Aeschlimann A, Steiner W, Stucki G. Responsiveness of the WOMAC osteoarthritis index as compared with the SF-36 in patients with osteoarthritis of the legs undergoing a comprehensive rehabilitation intervention. Ann Rheum Dis. 2001;60(9):834–40.
Bennell KL, Egerton T, Martin J, Abbott JH, Metcalf B, McManus F, et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311(19):1987–97.
Lutz J, Offidani E, Taraboanta L, Lakhan SE, Campellone TR. Appropriate controls for digital therapeutic clinical trials: a narrative review of control conditions in clinical trials of digital therapeutics (DTx) deploying psychosocial, cognitive, or behavioral content. Front Digital Health. 2022;4:823977.
Acknowledgements
The authors thank David Seissler and Jonas Albert from fbeta for the support and advice with study planning. We further acknowledge support by the Springer Nature Author Services for editing the manuscript for proper English language, grammar, punctuation, spelling, and overall style.
Dissemination policy
The University Hospital Tübingen has a basic right of publication of study data. The study protocol and results will be communicated at scientific congresses and/or in peer-reviewed scientific journals. The sponsor Sporlastic GmbH has the right to make the study results public as a part of corporate communications.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is financed by Sporlastic GmbH (Nürtingen, Germany). Sporlastic GmbH also provided the digital application and sensors that are used in the clinical trial. The trial sponsor Sporlastic GmbH has a contractional relationship with Kineto Tech Rehab SRL. Kineto Tech Rehab SRL is the manufacturer of the product (sensors & app). Sporlastic is the exclusive distribution partner for the contract area (Germany). We further acknowledge support by Open Access Publishing Fund of the University of Tübingen.
Author information
Authors and Affiliations
Contributions
IK, VD, PM and PJ contributed to the conception and design of the study. VD drafted the manuscript. IK, PM and PJ critically revised the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The complete final trial dataset will only be assessed by employees of the University Hospital Tübingen. Any information provided in the context of the interaction with the digital application (technical queries, log files, information given within the scope of the software use) can be assessed by the app producer and the study sponsor. However, these data are pseudonymized and cannot be reidentified by any party other than the University Hospital.
This study is approved by the Ethics Committee of the Medical Faculty of the University of Tübingen (688/2022BO2). The trial was registered on 20/01/2023 at www.drks.de (ID: DRKS00030932). Participants will be informed about the potential harms and benefits of the study and the voluntariness of study participation. Written informed consent will be obtained from all participants prior to study inclusion. In case of modifications of the protocol, an amendment will be sent to the local ethics committee prior to the implementation.
Consent for publication
The person in Fig. 1 gave written informed consent for the image to be included in this manuscript.
Competing interests
The exercise intervention that is implemented in the re.flex app was designed by employees of the University Hospital (i.e., VD, IK) in the context of a previous cooperation project between the trial sponsor Sporlastic GmbH and the University Hospital Tübingen. The pilot study of this trial protocol was conducted during this cooperation project as well. The University Hospital will be appropriately compensated for granting rights of use to the results generated by employees of the University Hospital (intellectual property) in the event of successful commercialization of the app. University Hospital will compensate the employees (i.e., VD, IK) who were involved in the previous collaboration and are authors of the intellectual property from these revenues. Contract negotiations are currently underway. However, the PI (PJ) of the study outlined in this study protocol and the biomedical statisticians (PM) do not have any conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Reporting checklist for protocol of a clinical trial (SPIRIT guidelines).
Additional file 2.
The TIDieR (Template for Intervention Description and Replication) Checklist.
Additional file 3.
Consent form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dieter, V., Martus, P., Janssen, P. et al. Evaluation of a 12-week app-guided exercise intervention in patients with knee osteoarthritis (re.flex): a study protocol for a randomized controlled trial. BMC Digit Health 1, 43 (2023). https://doi.org/10.1186/s44247-023-00040-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44247-023-00040-1