Abstract
Mechanical ventilation is a life-saving technology, but it can also inadvertently induce lung injury and increase morbidity and mortality. Currently, there is no easy method of assessing the impact that ventilator settings have on the degree of lung inssflation. Computed tomography (CT), the gold standard for visually monitoring lung function, can provide detailed regional information of the lung. Unfortunately, it necessitates moving critically ill patients to a special diagnostic room and involves exposure to radiation. A technique introduced in the 1980s, electrical impedance tomography (EIT) can non-invasively provide similar monitoring of lung function. However, while CT provides information on the air content, EIT monitors ventilation-related changes of lung volume and changes of end expiratory lung volume (EELV). Over the past several decades, EIT has moved from the research lab to commercially available devices that are used at the bedside. Being complementary to well-established radiological techniques and conventional pulmonary monitoring, EIT can be used to continuously visualize the lung function at the bedside and to instantly assess the effects of therapeutic maneuvers on regional ventilation distribution. EIT provides a means of visualizing the regional distribution of ventilation and changes of lung volume. This ability is particularly useful when therapy changes are intended to achieve a more homogenous gas distribution in mechanically ventilated patients. Besides the unique information provided by EIT, its convenience and safety contribute to the increasing perception expressed by various authors that EIT has the potential to be used as a valuable tool for optimizing PEEP and other ventilator settings, either in the operative room and in the intensive care unit. The effects of various therapeutic interventions and applications on ventilation distribution have already been assessed with the help of EIT, and this document gives an overview of the literature that has been published in this context.
Similar content being viewed by others
Introduction
Electrical impedance tomography (EIT) is a non-invasive tool that displays regional changes in lung volume and the distribution of ventilation at the bedside in real-time, providing information about gas distribution, regional ventilation delay, and, more recently pulmonary perfusion. This dynamic assessment can help clinicians optimize and individualize ventilator parameters tailored to a patient’s characteristics. Indeed, EIT may assist in optimizing mechanical ventilation settings, taking into account the heterogeneity of the lung. In addition, real-time monitoring of lung function can allow clinicians to more accurately predict patient recovery, thereby reducing dependence on the ventilator, and at the same time, avoiding the risks of premature weaning. Being a noninvasive and safe technique, the popularity of EIT is increasing among phyisicians caring for mechanically ventilated patients. This review article provides an overview of the EIT literature with a focus on its application in various clinical scenarios.
Acute respiratory distress syndrome
In the 1980s, preliminary reports discussed how impedance changes measured within the thorax during spontaneous breathing with an EIT system were linearly correlated with tidal volume [1]. As it became clear that the acute respiratory distress syndrome (ARDS) was characterized by a heterogeneous distribution of ventilation [2], EIT emerged as a practical tool to monitor regional ventilatory abnormalities and guide the titration of mechanical ventilation in patients with ARDS [3,4,5].
Assess recruitability
Recruitability refers to the potential to stabilize the re-opening of atelectatic lung regions with the application of higher positive end-expiratory pressure (PEEP). The observation that ARDS patients present highly variable degrees of recruitability [6] has fueled interest in measuring recruitability at the bedside and suggests that this should be done prior to selecting a personalized PEEP. Increasing PEEP in the absence of recruitability is associated with deleterious consequences including hemodynamic depression and overdistension. A simple bedside maneuver based on respiratory mechanics can estimate recruitability with higher PEEP [7], but compared to EIT-based methods, this technique cannot be used during spontaneous breathing or for continuous monitoring and only provides a global assessment.
The change in end-expiratory lung impedance measured by EIT allows for the calculation of the volume recruited by changing PEEP [8]. This volume is then divided by the change in PEEP to obtain its compliance. Finally, compliance of the recruited lung is divided by the compliance of the respiratory system at lower PEEP (i.e., the size of the baby lung) to obtain the recruitment to inflation (R/I) ratio. Values above 0.5–0.7 indicate higher recruitability. The regional R/I ratio of the dorsal lung may be even more sensitive for the estimation of recruitability.
Importantly, EIT assesses recruitability at a regional level and provides crucial information about whether recruitment in the dorsal lung has occurred at the cost of concomitant overdistension in the ventral areas. EIT has shown heterogeneity in regional lower inflection points (LIP) and upper inflection points (UIP) of pressure-volume curves in ARDS patients [9, 10], with higher regional LIP indicating need for higher PEEP to restore aeration and airway patency.
Finally, improved compliance of the most dorsal regions at higher PEEP may indicate recruitability in a dynamic and simple fashion [11].
Setting positive end-expiratory pressure
In 1975, Suter et al. proposed that the optimal PEEP resulted in the highest respiratory system compliance, the lowest dead-space fraction, and the greatest oxygen delivery [12]. In the era of lung protective ventilation, the benefits of improved oxygen transport must be balanced with the risks of local alveolar overdistension and ventilator-induced lung injury [13].
Costa et al. described an EIT-based approach to assess collapse and overdistension by comparing EIT to CT scanning [14]. By assessing the tidal change in lung impedance divided by the driving pressure they proposed a “per pixel” compliance. The relative changes in pixel compliance during a decremental PEEP trial allowed quantification of the regional effects of PEEP on lung mechanics. Loss of compliance associated with increasing PEEP was termed “overdistension” whereas loss of compliance associated with decreasing PEEP was termed “collapse.” Knowing the percentage of overdistended and collapsed pixels at each level, optimal PEEP was defined as the value that maximized recruitment and minimized overdistension. This approach often provides an incentive to reduce PEEP [15].
Once PEEP is set, EIT can ensure that recruitment is maintained. Eronia et al. [16] performed a PEEP titration targeting stability of end-expiratory lung volume. This method defined a PEEP that maintained lung recruitment in most patients and led to the selection of higher PEEP compared to a standard PEEP to FiO2 table. This approach was also associated with an improvement in respiratory system compliance and oxygenation.
An important caveat to EIT is that it provides measurements of lung volume that are based on relative values between two states. Furthermore, the range of start and end PEEP values included in the decremental trial can potentially mislead the clinician if too narrow a range is used and impact the EIT-derived optimal PEEP. The following case study show the an integrated approach used to identify an appropriate setting of ventilation.
Case study
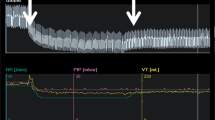
A 35-year-old man was admitted to the ICU with a diagnosis of ARDS secondary to COVID-19. He was sedated and paralyzed while receiving controlled mechanical ventilation with a volume-controlled mode. The baseline total PEEP was 14 cmH2O. Airway driving pressure was 14 cmH2O and exhaled VT was 429 mL with a RR of 2 23 and FiO2 of 0.4. The R/I ratio was calculated as 0.44, suggesting a lower potential for lung recruitment at higher PEEP. A decremental PEEP trial was performed to personalize this patient’s PEEP. The ventilator mode was switched to pressure-control (PC) with a driving pressure (PC above PEEP) set at 15 cmH2O throughout the trial. First, PEEP was increased to 18 cmH2O (time point A on Fig. 1) and maintained for 1 minute. Then PEEP was decreased by increments of 2 cmH2O until a PEEP of 8 cmH2O was achieved, with each PEEP level maintained for 1 minute (time points B through F). Respiratory mechanics and oxygen saturation recorded during each step reported in Table 1.
demonstrates a regional analysis of lung mechanics obtained during the decremental PEEP trial, provided as a diagnostic tool. Alveolar overdistension is represented in orange as a “compliance loss” (C loss HP) that occurred at higher PEEP (more overdistension at time point A (PEEP 18 cmH2O) compared to B (PEEP 16 cmH2O)). Alveolar collapse is represented in white as a “compliance loss” (C loss LP) that occurred at lower PEEP (more collapse at time point F (PEEP 8 cmH2O) compared to E (PEEP 10 cmH2O)). The crossover point representing minimal overdistension and atelectasis was 10 cmH2O (time point E). Below this value, dorsal collapse increased. Above this value, no collapse was detected, but increasing ventral overdistension developed. This example illustrates how EIT might be used as an incentive to reduce PEEP and optimize regional lung mechanics in a patient with ARDS
Non-intubated patients
There is increasing interest in preserving spontaneous breathing in patients with ARDS and understanding whether such an approach is lung protective [17]. In this setting, EIT can assist in titrating respiratory support and identifying the consequences of excessive respiratory drive and effort that increase regional lung stress and strain [18, 19].
Yoshida et al. used EIT to demonstrate that a switch to spontaneous breathing in an ARDS patient could result in a nearly twofold increase in ventilation distributed to dependent lung zones [20]. This was accompanied by deflation of the ventral lung shortly after initiation of the breath. This movement of air from one region of the lung to another, termed “occult pendelluft”, can cause significant local overdistension.
In ARDS patients treated with pressure support ventilation, Mauri et al. used EIT to demonstrate a more homogenous distribution of ventilation when the pressure support level was titrated to a physiologic range of P0.1 [21]. EIT can also be used to assess the clinical impact of respiratory support with high flow nasal cannula [22] and identify patients at risk for failure during support with non-invasive ventilation [23].
Acute exacerbations of COPD
Chronic obstructive pulmonary disease (COPD) is characterized by a heterogenous increase of regional airway resistances and lung compliances. In stable patients with COPD, this pathologic increase in regional time constants can be visualised by EIT as a heterogeneous distribution of ventilation and variable filling/emptying times across lung units [24, 25]. Acute exacerbations of COPD (AECOPD) are a frequent cause of presentation to the emergency department and clinicians face the difficult challenge of balancing effective CO2 washout with the risk of dynamic hyperinflation when providing respiratory support. EIT can aid in monitoring and managing these patients, particularly in titrating ventilatory support and assessing response to therapy (Table 2).
In ventilated patients with AECOPD, dynamic hyperinflation (intrinsic PEEP) can occur due to the increased time constants of lung units and shorter expiratory time due to a higher respiratory rate. If the externally applied PEEP is inadequate, intrinsic PEEP results in increased work of breathing and an inhomogeneous distribution of ventilation. In this setting, EIT can be used to set an optimal PEEP that minimizes the consequences of dynamic hyperinflation. Interestingly, Kostakou et al. performed a PEEP titration assessing ventilation heterogeneity using EIT in a mechanically ventilated patient with a severe AECOPD and evidence of dynamic hyperinflation [26]. They set PEEP to 0%, 50%, 80%, 100%, and 150% of the globally measured intrinsic PEEP and measured the regional delay of ventilation, the time for a lung region to attain a certain impedance change [32]. An optimal homogeneity with the lowest delay of ventilation was achieved at a PEEP set to 80% of the intrinsic PEEP. Interestingly, this PEEP value also resulted in the greatest exhaled tidal volume, but not the greatest respiratory system compliance.
In an intubated patient with COPD, Mauri et al. demonstrated the usefulness of EIT in selecting a personalized external PEEP in the setting of intrinsic PEEP [27]. During a decremental PEEP trial, EIT displayed that the PEEP level at which dependent lung regions stopped deflating (indicative of the quantity of regional intrinsic PEEP) was higher than for non-dependent. PEEP was set at a level corresponding to the highest level of regional intrinsic PEEP, and the patient was successfully transitioned to assisted ventilation. Importantly, this PEEP level was higher than the traditionally measured global value obtained during an end-expiratory occlusion.
Karagiannidis et al. demonstrated the feasibility and reliability of measuring regional time constants using EIT [31]. They reported significant heterogeneity and overall increased time constants in invasively ventilated patients with AECOPD compared to ARDS. Moreover, they detected regional differences in airflow limitation and the response to different levels of applied PEEP.
The heterogeneity of time constants in patients with COPD can cause an asynchronous pattern of ventilation giving rise to occult pendelluft and regional overdistension. In patients with AECOPD, Sang et al. demonstrated significant heterogeneity in the magnitude and timing of impedance versus time curves in different regions of the lung [33]. These “phase shifts” and the heterogeneity of amplitude differences indicated delays between emptying of different lung units. The magnitude of EIT measured expiratory delays worsened with increasing airway resistance and improved after administration of bronchodilator therapy, suggesting that EIT can be a helpful adjunct in monitoring patients with AECOPD over time.
By measuring flow versus time curves at end-expiration, Zhao et al. also used EIT to identify regional air-trapping and assess the response to bronchodilator therapy in patients with AECOPD [28].
In terms of ventilation mode, in patients with AECOPD receiving assisted (pressure support) ventilation, Sun et al. used EIT to demonstrate that switching to a neurally adjusted ventilatory assist mode increased the homogeneity of the distribution of ventilation and reduced the work due to trigger [30].
Altogether, a nuanced approach to ventilator management and a PEEP selection that optimizes work of breathing in patients with AECOPD may be facilitated with an EIT-guided approach.
COVID-19 acute respiratory failure
The novel coronavirus disease 2019 (COVID-19) pandemic has led to an overwhelming amount of mechanically ventilated patients [34] with severe hypoxemic acute respiratory failure (hARF) consequent to either alveolar or vascular injury or both [35]. EIT has been proposed as a valuable tool to personalize the management of COVID-19 patients with hARF [36,37,38,39,40].
Recent data indicate that limiting driving pressure (DP) as much as possible reduces the risk of death in mechanically ventilated COVID-19 hARF patients [41]. By estimating the loss of compliance due to lung collapse and overdistension [14], EIT offers the possibility to minimize DP by individualizing PEEP selection [42].
Sella et al. [38], in a cohort of intubated COVID-19 patients, found that the median PEEP selected by EIT (PEEPEIT) that minimized the overall loss of compliance was 12 cmH2O [interquartile range 10–14 cmH2O] [43] and corresponded to the intersection between the EIT alveolar collapse and overdistension curves [14]. Notably, the loss of lung compliance due to lung collapse observed with PEEP values from the lower PEEP/FiO2 table was comparable to PEEPEIT, whereas the loss of lung compliance due to lung overdistension was significantly greater with PEEP values from the higher PEEP/FiO2 table than with PEEPEIT, suggesting better agreement between PEEPEIT and the lower PEEP/FiO2 table [38]. In keeping with these results, Perier et al., in a series of 17 COVID-19 hARF patients, found a median PEEPEIT of 12 [9-12] cmH2O, without significant differences between patients with respiratory system compliance (Crs) ≥ 40 mL/cmH2O and <40 mL/cmH2O [39].
In contrast, Van der Zee et al. found higher values of PEEPEIT (21 [16–22] cmH2O), closer to those advised by the higher PEEP/FiO2 table [40]. These discrepancies may be partly explained by the different criteria used for PEEPEIT selection in this study, set 2 cmH2O above the intersection of the curves representing the cumulative percentage of compliance loss due to lung collapse and overdistension [40]. Furthermore, Van der Zee et al. enrolled more obese patients (median body mass index of 30.0 [27.0–34.0] kg/m2 [40], compared to Sella et al. (26.2 [25.4–30.9] kg/m2) [38], perhaps explaining the higher PEEP in the setting of reduced chest wall compliance [44].
A scientific dispute among opinion leaders has debated the use of noninvasive respiratory supports in COVID-19 hARF. While some authors are concerned about the risk of patient self-inflicted lung injury [45], others are cautious, considering the harms of unnecessary intubation [46]. Indeed, duration of NIV use [47, 48] and location of application [48] have been associated with hospital mortality in COVID-19 patients intubated after NIV failure. EIT has been proposed as a tool to assess the response to continuous positive airway pressure (CPAP) and recognize patients at risk for CPAP failure [23]. In a series of 10 patients admitted to the ICU for COVID-19 pneumonia and supported with CPAP, Rauseo et al. performed an EIT-guided decremental PEEP trial from 12 cmH2O to 6 cmH2O and found that a reduction of EELI smaller than 40% after PEEP de-escalation predicted CPAP failure [23].
COVID-19 hARF is characterized not only by alveolar injury, but also by severe pulmonary vascular disruption [49] with small- and mid-sized pulmonary vessel thrombosis [35], associated with a hypercoagulable state [50, 51]. Recent data from COVID-19 patients suggest the potential of an EIT perfusion assessment to detect both ventilation-perfusion (V/Q) mismatch [52,53,54] and pulmonary vasculature alterations, consistent with findings of computed tomography pulmonary angiography [55, 56].
Prone positioning has been widely applied in COVID-19 hARF, with 61% of intubated patients undergoing at least one cycle of prone positioning [56].
Nevertheless, the mechanisms underlying the improvement in oxygenation after prone positioning in COVID-19 patients remain unclear.
Zarantonello et al. described the case of one COVID-19 hARF patient, studied with EIT ventilation-perfusion analysis in the supine position and 60 min after being turned prone, and found that prone positioning increased ventilation in the dorsal areas and shifted perfusion to the ventral areas, overall improving V/Q matching [52]. Perier et al., in 17 COVID-19 hARF patients [39], found no difference between PEEPEIT in the supine and prone position and no improvement in DP and Crs after turning patients prone, thereby casting doubt on the role of alveolar recruitment in the improvement of arterial oxygenation during prone positioning. Subsequently, Perier et al., in a cohort of 9 patients with COVID-19 hARF, showed that turning patients from the supine to prone position decreased ventral dead space and dorsal shunt with a trend towards an improvement in V/Q matching, especially in the ventral areas of the lung [53].
Anesthesia and perioperative period
EIT monitoring during the perioperative period can improve the care of patients undergoing different surgical procedures and aims to reduce post-operative pulmonary complications by identifying factors that may benefit from a personalized ventilator strategy.
Anesthesia induction
A certainty of anesthesia induction is a decrease in functional residual capacity, the magnitude of which is unpredictable (FRC) [57, 58]. Ideal ventilator management aims to maintain end-expiratory lung volume at a value that is as similar as possible to preoperative FRC. Reductions in FRC result in derangements of the blood gases and ventilation/perfusion mismatch [59,60,61].
On the other hand, an unphysiological increase in FRC should be considered unsafe, as this may augment lung stress [62]. Difficulty in providing an optimal ventilator strategy is probably due to the limited value of information available during perioperative ventilation, such as plateau pressure or tidal volume per kilogram of body weight, which can only weakly characterize the mechanical properties of the respiratory system [63]. Furthermore, with the adoption of “protective ventilation” in the operative room, the modern concept of driving pressure finds its application, only a very low external PEEP is given, for reliable information about dynamic strain [64]. Based on the recent literature, EIT monitoring may play a pivotal role in providing a bedside assessment of FRC.
EIT demonstrated a decrease in FRC after anesthesia induction that was reversed by the application of PEEP in morbidly obese patients undergoing laparoscopic gastric bypass surgery [65]. In these patients, pre-oxygenation with a tight-fitting mask and 10 cmH2O of PEEP transiently increased FRC and prevented hypoxemia during anesthesia induction. Nestler et al. demonstrated that bag-mask ventilation without applied PEEP resulted in a significant decrease in post-intubation FRC [66]. Therefore, EIT represents an encouraging opportunity to monitor the efficacy of different pre-oxygenation strategies (Fig. 2).
Intraoperative mechanical ventilation
As mentioned above, the loss of FRC during general anesthesia is unpredictable, due to many contributing factors including a patient’s pathophysiology, anesthesia technique, body positioning, and/or surgical procedures. It is, however, well known that this loss of FRC results in atelectasis in more than 90% of patients [57,58,59]. Ukere et al. [67, 68], using EIT in both anesthetized and awake patients identified under ventilated “silent spaces,” in different body positions. The availability of this information at the bedside may guide the setting of external PEEP, limiting the development of atelectasis and consequences related to PPC and infections. During general anesthesia, based on a breath-by-breath analysis, electrical impedance tomography can highlight changes in lung aeration and the distribution of ventilation, thereby allowing the clinician to titrate mechanical ventilation on the basis of a patient’s regional respiratory mechanics [69]. In the last few years, research based on optimizing PEEP guided by EIT at the bedside identified a method for determining the “best” PEEP as that which minimizes alveolar overdistension and collapse, limits driving pressure and optimizes oxygenation [70]. Indeed, Pereira et al. showed that the effect of this EIT-based approach was more considerable during laparoscopic procedures compared to laparotomies. Nestler et al. [66] proposed another EIT-derived parameter to set PEEP in obese patients undergoing general anesthesia: the regional ventilation delay index (RVDI), defined as the standard deviation of Regional Ventilation Delay (a measure of the temporal delay in ventilation of regions of the lung) in all pixels. A lower RVDI indicates a more homogeneous distribution of ventilation and thus may limit derecruitment. Their protocol consisted of a recruitment maneuver, followed by the application of PEEP titrated to minimize RVDI. When compared to a fixed PEEP (5 cmH2O), this method led to a significant improvement in oxygenation and better regional homogeneity, with no deleterious postoperative effects.
One of the most useful and fascinating parameters obtained from EIT to evaluate the effects of different PEEP levels is the End Expiratory Lung Impedance (EELI), which represents the impedance at the end of expiration. Changes in EELI reflect lung recruitment due to PEEP. Using this parameter, Erlandsson et al. [65] showed that, in obese patients undergoing laparoscopic surgery, an increase or decrease in the slope of EELI following a change in PEEP, corresponded to recruitment or derecruitment, respectively, whereas a stable end-expiratory lung volume reflected optimal PEEP. Eronia et al. [16], in patients with acute respiratory failure, showed that it is possible to measure recruitment from the variation in EELI measured at the beginning and end of a recruitment maneuver and that PEEP could be titrated according to the change in ∆EELI. This method might even be useful during general anesthesia: an increase in EELI lower than that predicted by the recruited volume could be helpful in suggesting the presence of lung overdistension [71].
EIT application does not exclusively describe how to set ventilation or physiology of the respiratory system. It has also been used during thoracic surgery to confirm the correct positioning of double lumen endotracheal tubes (DLT) and titrate the optimal combination of tidal volume and PEEP in patients requiring one-lung ventilation (OLV). Compared to the gold standard fiberoptic bronchoscopy for routine confirmation of the correct positioning of DLT, EIT has the advantage of allowing clinicians to non-invasively identify any misplacement of DLTs in the contralateral main bronchus by accurately displaying left and right lung ventilation [72]. Transitioning from two-lung ventilation (TLV) to OLV, the mechanical properties of the ventilated lung undergo significant changes. The exclusion of one lung from ventilation in the lateral decubitus position determines a change in lung compliance, resistance, and the distribution of tidal volume. Hence, the ventilatory management of these patients is challenging and tidal volume and PEEP should be frequently reassessed during each surgery step [60, 73]. Each of these physiologic variations can be detected by EIT. Using an index of inhomogeneity derived from EIT (GI, global inhomogeneity index), Zhao et al. [74] showed that it is possible to titrate the combination of PEEP and TV in patients shifting from TLV to OLV. In their study, they found a good degree of inter-patient equivalence and the GI correlated with the gas distribution in the lung. The same authors recently explored if the regional ventilation distribution (measured by EIT) and PaO2 could help titrating TV and PEEP during OLV [75].
Future perspectives and large RCTs will elucidate the usefulness of EIT in setting intraoperative ventilation and clarify whether the use of EIT reduces the incidence of postoperative pulmonary complications (PPC).
Post-extubation period
EIT also allows for the continuous monitoring of patients in the postoperative period (Fig. 3). The end of surgery and subsequent post-extubation phase are times that require close monitoring due to the abrupt discontinuation of mechanical ventilation and loss of respiratory monitoring that was provided by the ventilator. In addition, these changes coincide with the persisting consequences of sedation, including muscle weakness, reduced inspiratory effort and transpulmonary pressure, an impaired cough reflex and ability to clear secretions due to residual paralysis, and/or poor pain control. Altogether, these factors contribute to an increased risk of postoperative pulmonary complications, which might easily be identified by monitoring changes in ventilation distribution and EELI measured by EIT, especially at the end of the surgical procedure. Schaefer et al. [69] described the feasibility of using EIT to monitor regional tidal volume distribution before the induction of anesthesia, intra-operatively, after extubation, and in the post-operative period. The authors showed that during general anesthesia, tidal ventilation is distributed to the ventral part of the lungs due to muscle paralysis. When spontaneous breathing is restored and following extubation, ventilation, and re-aeration of the dorsal part of the lungs take place, increasing the homogeneity of ventilation, decreasing the tendency for atelectasis. Interestingly, despite using a personalized intra-operative PEEP setting and a recruitment maneuver before extubation, early post-operative EELV is lower compared to baseline before induction of anesthesia [66]. A decrease in EELV at the end of surgery might represent an “alarm bell” that suggests an increased risk of developing postoperative atelectasis and extubation failure. For this reason, EIT monitoring can help to identify patients with a reduced post-operative EELV who might benefit from post-extubation non-invasive ventilation and early mobilization (i.e., obese patients) [76]. Karsten et al. used EIT to evaluate the impact of low versus high PEEP during laparoscopic surgery on post-operative ventilation distribution and showed that a higher intra-operative PEEP resulted in a more homogeneous distribution of ventilation in the early post-operative period [77].
Weaning
Weaning is the entire process leading patients to the discontinuation of mechanical ventilation and extubation [78]. A spontaneous breathing trial (SBT) is commonly performed to determine whether weaning has been successful and the patient is ready for extubation. While various clinical parameters are utilized to define SBT success, the most powerful predictor of weaning success is the respiratory rate (RR) to tidal volume (Vt) ratio (RR/Vt) [78]. About one fifth of patients, with rates varying from 14 to 31% among studies, fail their first SBT attempt and require reinstitution of mechanical ventilation [78]. After a successful SBT, a fraction of patients, ranging from 3 to 19% among studies, develop post-extubation respiratory failure requiring re-intubation, a complication associated with significantly increased mortality [78]. Prophylactic application of non-invasive ventilation (NIV) soon after extubation in patients at risk of post-extubation respiratory failure may prevent the need for re-intubation and improve outcomes [79]. Most studies broadly consider at-risk patients to be those older than 65 years old or with underlying cardiac or respiratory disease [79]. It is, therefore, of paramount clinical importance to improve the precision of weaning and extubation failure predictions and recent studies indicate a role of EIT for this purpose.
In a general population of 42 mechanically ventilated patients, Lima et al. assessed the variation of end-expiratory lung impedance (EELI) occurring during a 30-min SBT, as conducted by T-piece (10 patients) or low levels of pressure-support ventilation (PSV) (32 patients). In the T-piece group, irrespective of SBT outcome, EELI progressively declined throughout the SBT, though a significantly greater decrease in EELI was observed in patients failing the SBT [80]. In the PSV group, EELI did not vary significantly during the SBT and no difference in EELI variations was observed between patients with different SBT outcomes, likely because ventilator settings, including PEEP, before and during the SBT were quite similar [80]. No difference in Tidal Impedance Variation (TIV) was observed in both groups [80].
In 78 patients at risk for extubation failure, Longhini et al. applied EIT during an SBT conducted with low (2 cmH2O) CPAP applied through the ventilator circuit [81]. The authors also assessed the heterogeneity of air distribution within the lung, using the Global Inhomogeneity index (GI) [81]. Compared to weaning successes, patients failing the SBT were characterized by a greater loss in EELI during the SBT and a greater GI at baseline and during the course of the SBT [81]. Again, no difference in TIV was observed between SBT successes and failures [81].
In 31 patients experiencing prolonged weaning, Bickenbach et al. also reported that T-piece SBT failure was characterized by a greater GI at baseline, while gas exchange and RR/Vt were not different between patients succeeding and failing the SBT [82]. Their results suggest that not attempting a SBT in patients with a baseline GI > 41.5 would avoid 87.5% of all SBT failures [82]. Moon et al. recently found that GI was significantly greater in patients failing a T-piece SBT, in a population of 40 patients either with (n=16) or without (n=24) diaphragm dysfunction [83]. In keeping with these previous results [81,82,83], in 53 patients mechanically ventilated for more than 72 h and undergoing their first T-piece SBT, Wang et al. further confirmed that GI prior to SBT helps to predict SBT outcome [84].
In a cohort of 30 patients with prolonged weaning, Zhao et al. described different patterns of ventilation according to weaning outcomes; in patients succeeding, ventilation was redistributed towards the dorsal regions, with a more homogeneous distribution between the anterior and posterior regions when decreasing support levels [85].
During the weaning process, Longhini et al. demonstrated that chest physiotherapy as applied by high-frequency chest wall oscillation (HFCWO) improves lung aeration in patients with copious secretions. Also noteworthy, the association of HFCWO with a recruitment maneuver did not produce any further physiological benefit [86].
Finally, the study by Longhini et al. was the only one that investigated the potential of EIT to predict the need for NIV in the post-extubation period. Among 61 patients who successfully passed a SBT, 22 (36.1%) experienced post-extubation respiratory failure within 48 h. Up to 30 min after extubation, no differences in EELI, TIV, or GI were observed between patients succeeding and failing extubation [81].
Patient-ventilator dyssynchrony
In order to avoid the consequences of potentially harmful ventilator asynchronies, EIT monitoring used during assisted spontaneous breathing can facilitate the early recognition of breath stacking and pendelluft [87, 88].
Breath stacking may be caused by reverse triggering or double-triggering and results in consecutive inspiratory cycles delivered by the ventilator during an incomplete exhalation [87]. When breath stacking occurs, EIT can demonstrate potentially harmful end-inspiratory lung volumes and is more sensitive when compared to conventional monitoring, which only indicates a modest increase in VT [89].
Pendelluft describes how gas movement between different pulmonary regions results in an uncontrolled and dangerous alveolar de-inflation and inflation, depicted as intrapulmonary asynchrony. EIT allows for the monitoring of a pendelluft phenomenon, since it can readily display air movement within the lung from nondependent to dependent regions even when VT is unchanged [88]. This phenomenon can be caused by excessive diaphragmatic contractions during strong spontaneous efforts and may increase strain of the dependent lung during early inflation. Conventionally monitored respiratory parameters such as flow, volume, and pressure are unable to demonstrate Pendelluft whereas EIT can [88].
Noninvasive ventilation
During non-invasive ventilation, global parameters such as pressure-volume curves or the respiratory system compliance do not reliably illustrate what is actually happening in the lung, especially regarding the regional distribution of the administrated tidal volume, where high tidal volumes may lead to patient self inflicted lung injury (PSILI) [90,91,92].
A lung protective strategy, either during invasive and noninvasive ventilation, should require real-time monitoring of regional lung ventilation to determine the distribution of lung ventilation such as hyperventilation. During the height of the COVID-19 pandemic, Rauseo et al. [23] demonstrated that the number of patients with respiratory failure far exceeded the availability of intensive care unit beds, often prompting physicians to choose non-invasive ventilation as initial therapy. Under such conditions, EIT was identified as a helpful tool to assess the response of patients to NIV and rapidly identify an optimal ventilatory strategy.
Bordes et al. [93] assessed functional residual capacity and ventilation distribution in eighteen spontaneously breathing adult patients undergoing digestive endoscopic procedures under anesthesia and showed that, in awake patients, tidal volume was primarily distributed to the dependent lung (57.5 vs 43.1%; P = .009), whereas after anesthesia induction, ventilation shifted to the nondependent lung (43.1% before anesthesia, 58.9% after anesthesia; P = .002) with a marked decrease in end-expiratory lung impedance. In the same cohort, application of noninvasive ventilation resulted in a significant increase in end-expiratory lung impedance (P = .005) without changing the distribution of ventilation.
Lastly, high flow nasal cannula (HFNC) under EIT monitoring has shown improved oxygenation by increasing both end-expiratory lung volume and tidal volume, regardless of body position suggesting an increase in functional residual capacity [94, 95].
Given the high number of patients treated with NIV/HFNC, large randomized controlled trials are needed; future applications of EIT monitoring could be in thoracic trauma patients and/or pre- and postoperative patients treated with prophylactic NIV/HFNC (to avoid intubation and or complications related to intubation).
Conclusions
Evidence-based medicine has demonstrated that “one size doesn’t fit all.” Lung monitoring and mechanical ventilation have been enhanced by the use of the EIT system, and despite its limitations, this device represents a remarkable technological advance in the fields of anesthesia and critical care medicine.
Future perspectives
Future areas of inquiry include using EIT to guide the use of more novel technologies such as helmet NIV or extra-corporeal CO2 removal in patients with AECOPD.
Beyond ventilation, a novel feature of EIT is the ability to obtain the distribution of pulmonary blood flow [96, 97] and a pixel-based mapping of ventilation to perfusion ratios. This technology represents an exciting future potential to guide the treatment of patients with ARDS.
Availability of data and materials
Not applicable.
Change history
30 June 2022
A Correction to this paper has been published: https://doi.org/10.1186/s44158-022-00059-2
References
Harris ND, Suggett AJ, Barber DC, Brown BH (1988) Applied potential tomography: a new technique for monitoring pulmonary function. Clin Phys Physiol Meas 9 Suppl A:79–85
Gattinoni L, Presenti A, Torresin A, Baglioni S, Rivolta M, Rossi F, Scarani F, Marcolin R, Cappelletti G (1986) Adult respiratory distress syndrome profiles by computed tomography. J Thorac Imaging 1:25–30
Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, Tanaka H, Sipmann FS, Santos DC, Barbas CS, Carvalho CR, Amato MB (2004) Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med 169:791–800
Frerichs I, Amato MB, van Kaam AH, Tingay DG, Zhao Z, Grychtol B, Bodenstein M, Gagnon H, Bohm SH, Teschner E, Stenqvist O, Mauri T, Torsani V, Camporota L, Schibler A, Wolf GK, Gommers D, Leonhardt S, Adler A, group Ts (2017) Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 72:83–93
Bachmann MC, Morais C, Bugedo G, Bruhn A, Morales A, Borges JB, Costa E, Retamal J (2018) Electrical impedance tomography in acute respiratory distress syndrome. Crit Care 22:263
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, Sklar MC, Rauseo M, Ferguson ND, Fan E, Richard JM, Brochard L (2020) Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care Med 201:178–187
Mauri T, Eronia N, Turrini C, Battistini M, Grasselli G, Rona R, Volta CA, Bellani G, Pesenti A (2016) Bedside assessment of the effects of positive end-expiratory pressure on lung inflation and recruitment by the helium dilution technique and electrical impedance tomography. Intensive Care Med 42:1576–1587
Hinz J, Moerer O, Neumann P, Dudykevych T, Frerichs I, Hellige G, Quintel M (2006) Regional pulmonary pressure volume curves in mechanically ventilated patients with acute respiratory failure measured by electrical impedance tomography. Acta Anaesthesiol Scand 50:331–339
Scaramuzzo G, Spadaro S, Waldmann AD, Bohm SH, Ragazzi R, Marangoni E, Alvisi V, Spinelli E, Mauri T, Volta CA (2019) Heterogeneity of regional inflection points from pressure-volume curves assessed by electrical impedance tomography. Crit Care 23:119
Wolf GK, Gomez-Laberge C, Rettig JS, Vargas SO, Smallwood CD, Prabhu SP, Vitali SH, Zurakowski D, Arnold JH (2013) Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med 41:1296–1304
Suter PM, Fairley B, Isenberg MD (1975) Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 292:284–289
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136
Costa EL, Borges JB, Melo A, Suarez-Sipmann F, Toufen C Jr, Bohm SH, Amato MB (2009) Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 35:1132–1137
Yoshida T, Piraino T, Lima CAS, Kavanagh BP, Amato MBP, Brochard L (2019) Regional ventilation displayed by electrical impedance tomography as an incentive to decrease positive end-expiratory pressure. Am J Respir Crit Care Med 200:933–937
Eronia N, Mauri T, Maffezzini E, Gatti S, Bronco A, Alban L, Binda F, Sasso T, Marenghi C, Grasselli G, Foti G, Pesenti A, Bellani G (2017) Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: a feasibility study. Ann Intensive Care 7:76
Goligher EC, Dres M, Patel BK, Sahetya SK, Beitler JR, Telias I, Yoshida T, Vaporidi K, Grieco DL, Schepens T, Grasselli G, Spadaro S, Dianti J, Amato M, Bellani G, Demoule A, Fan E, Ferguson ND, Georgopoulos D, Guerin C, Khemani RG, Laghi F, Mercat A, Mojoli F, Ottenheijm CAC, Jaber S, Heunks L, Mancebo J, Mauri T, Pesenti A, Brochard L (2020) Lung- and diaphragm-protective ventilation. Am J Respir Crit Care Med 202:950–961
Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D (2020) Respiratory drive in critically ill patients. Pathophysiology and Clinical Implications. Am J Respir Crit Care Med 201:20–32
Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D (2020) Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 46:606–618
Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB (2013) Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 188:1420–1427
Mauri T, Bellani G, Confalonieri A, Tagliabue P, Turella M, Coppadoro A, Citerio G, Patroniti N, Pesenti A (2013) Topographic distribution of tidal ventilation in acute respiratory distress syndrome: effects of positive end-expiratory pressure and pressure support. Crit Care Med 41:1664–1673
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, Pesenti A (2017) Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 195:1207–1215
Rauseo M, Mirabella L, Laforgia D, Lamanna A, Vetuschi P, Soriano E, Ugliola D, Casiello E, Tullo L, Cinnella G (2021) A pilot study on electrical impedance tomography during cpap trial in patients with severe acute respiratory syndrome coronavirus 2 pneumonia: the bright side of non-invasive ventilation. Front Physiol 12:728243
Milne S, Huvanandana J, Nguyen C, Duncan JM, Chapman DG, Tonga KO, Zimmermann SC, Slattery A, King GG, Thamrin C (2019) Time-based pulmonary features from electrical impedance tomography demonstrate ventilation heterogeneity in chronic obstructive pulmonary disease. J Appl Physiol (1985) 127:1441–1452
Spinelli E, Dellino EM, Mauri T (2020) Understanding an unusual capnography waveform using electrical impedance tomography. Can J Anaesth 67:141–142
Kostakou E, Barrett N, Camporota L (2016) Electrical impedance tomography to determine optimal positive end-expiratory pressure in severe chronic obstructive pulmonary disease. Crit Care 20:295
Mauri T, Bellani G, Salerno D, Mantegazza F, Pesenti A (2013) Regional distribution of air trapping in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188:1466–1467
Zhao Z, Chang MY, Frerichs I, Zhang JH, Chang HT, Gow CH, Moller K (2020) Regional air trapping in acute exacerbation of obstructive lung diseases measured with electrical impedance tomography: a feasibility study. Minerva Anestesiol 86:172–180
Trenk F, Mendes L, Carvalho P, Paiva RP, Henriques J, Maglaveras N, Chouvarda I, Tsara V, Teixeira CA (2016) Evaluation of lung ventilation distribution in chronic obstructive pulmonary disease patients using the global inhomogeneity index. Annu Int Conf IEEE Eng Med Biol Soc 2016:5286–5289
Sun Q, Liu L, Pan C, Zhao Z, Xu J, Liu A, Qiu H (2017) Effects of neurally adjusted ventilatory assist on air distribution and dead space in patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care 21:126
Karagiannidis C, Waldmann AD, Roka PL, Schreiber T, Strassmann S, Windisch W, Bohm SH (2018) Regional expiratory time constants in severe respiratory failure estimated by electrical impedance tomography: a feasibility study. Crit Care 22:221
Muders T, Luepschen H, Zinserling J, Greschus S, Fimmers R, Guenther U, Buchwald M, Grigutsch D, Leonhardt S, Putensen C, Wrigge H (2012) Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury*. Crit Care Med 40:903–911
Sang L, Zhao Z, Yun PJ, Frerichs I, Moller K, Fu F, Liu X, Zhong N, Li Y (2020) Qualitative and quantitative assessment of pendelluft: a simple method based on electrical impedance tomography. Ann Transl Med 8:1216
Tonetti T, Grasselli G, Zanella A, Pizzilli G, Fumagalli R, Piva S, Lorini L, Iotti G, Foti G, Colombo S, Vivona L, Rossi S, Girardis M, Agnoletti V, Campagna A, Gordini G, Navalesi P, Boscolo A, Graziano A, Valeri I, Vianello A, Cereda D, Filippini C, Cecconi M, Locatelli F, Bartoletti M, Giannella M, Viale P, Antonelli M, Nava S, Pesenti A, Ranieri VM (2020) COVID-19 Northern Italian ICU Network. Use of critical care resources during the first 2 weeks (February 24-March 8, 2020) of the COVID-19 outbreak in Italy. Ann Intensive Care 10(1):133. https://doi.org/10.1186/s13613-020-00750-z PMID: 33044646; PMCID: PMC7549086
Calabrese F, Pezzuto F, Fortarezza F, Boscolo A, Lunardi F, Giraudo C, Cattelan A, Del Vecchio C, Lorenzoni G, Vedovelli L, Sella N, Rossato M, Rea F, Vettor R, Plebani M, Cozzi E, Crisanti A, Navalesi P, Gregori D (2021) Machine learning-based analysis of alveolar and vascular injury in SARS-CoV-2 acute respiratory failure. J Pathol 254(2):173–184. https://doi.org/10.1002/path.5653 Epub 2021 Mar 30. PMID: 33626204; PMCID: PMC8014445
Sella N, Pettenuzzo T, Zarantonello F, Andreatta G, De Cassai A, Schiavolin C, Simoni C, Pasin L, Boscolo A, Navalesi P (2021) Electrical impedance tomography: a compass for the safe route to optimal PEEP. Respir Med 187:106555. https://doi.org/10.1016/j.rmed.2021.106555 Epub 2021 Jul 30. PMID: 34352563
Zhao Z, Zhang JS, Chen YT, Chang HT, Hsu YL, Frerichs I, Adler A (2021) The use of electrical impedance tomography for individualized ventilation strategy in COVID-19: a case report. BMC Pulm Med 21(1):38. https://doi.org/10.1186/s12890-021-01411-y PMID: 33482796; PMCID: PMC7820832
Sella N, Zarantonello F, Andreatta G, Gagliardi V, Boscolo A, Navalesi P (2020) Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: electrical impedance tomography vs. PEEP/FiO2 tables. Crit Care 24(1):540. https://doi.org/10.1186/s13054-020-03242-5 PMID: 32873337; PMCID: PMC7459241
Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg AF, Razazi K, De Prost N, Amato M, Carteaux G, Mekontso DA (2020) Electrical impedance tomography to titrate positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome. Crit Care 24(1):678. https://doi.org/10.1186/s13054-020-03414-3 PMID: 33287864; PMCID: PMC7719729
van der Zee P, Somhorst P, Endeman H, Gommers D (2020) Electrical impedance tomography for positive end-expiratory pressure titration in covid-19-related acute respiratory distress syndrome. Am J Respir Crit Care Med 202(2):280–284. https://doi.org/10.1164/rccm.202003-0816LE PMID: 32479112; PMCID: PMC7365366
Boscolo A, Sella N, Lorenzoni G, Pettenuzzo T, Pasin L, Pretto C, Tocco M, Tamburini E, De Cassai A, Rosi P, Polati E, Donadello K, Gottin L, De Rosa S, Baratto F, Toffoletto F, Ranieri VM, Gregori D, Navalesi P, COVID-19 VENETO ICU Network (2021) Static compliance and driving pressure are associated with ICU mortality in intubated COVID-19 ARDS. Crit Care 25(1):263. https://doi.org/10.1186/s13054-021-03667-6 PMID: 34321047; PMCID: PMC8317138
Zhao Z, Chang MY, Chang MY, Gow CH, Zhang JH, Hsu YL, Frerichs I, Chang HT, Möller K (2019) Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care 9(1):7. https://doi.org/10.1186/s13613-019-0484-0 PMID: 30656479; PMCID: PMC6336593
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT (2004) National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351(4):327–336. https://doi.org/10.1056/NEJMoa032193 PMID: 15269312
Gattinoni L, Gattarello S, Steinberg I, Busana M, Palermo P, Lazzari S, Romitti F, Quintel M, Meissner K, Marini JJ, Chiumello D, Camporota L (2021) COVID-19 pneumonia: pathophysiology and management. Eur Respir Rev 30(162):210138. https://doi.org/10.1183/16000617.0138-2021 PMID: 34670808; PMCID: PMC8527244
Tobin MJ, Laghi F, Jubran A (2020) P-SILI is not justification for intubation of COVID-19 patients. Ann Intensive Care 10(1):105. https://doi.org/10.1186/s13613-020-00724-1 PMID: 32748116; PMCID: PMC7397710
Vaschetto R, Barone-Adesi F, Racca F, Pissaia C, Maestrone C, Colombo D, Olivieri C, De Vita N, Santangelo E, Scotti L, Castello L, Cena T, Taverna M, Grillenzoni L, Moschella MA, Airoldi G, Borrè S, Mojoli F, Della Corte F, Baggiani M, Baino S, Balbo P, Bazzano S, Bonato V, Carbonati S, Crimaldi F, Daffara V, De Col L, Maestrone M, Malerba M, Moroni F, Perucca R, Pirisi M, Rondi V, Rosalba D, Vanni L, Vigone F, Navalesi P, Cammarota G (2021) Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res 7(1):00541–02020. https://doi.org/10.1183/23120541.00541-2020 PMID: 33527074; PMCID: PMC7607967
Boscolo A, Pasin L, Sella N, Pretto C, Tocco M, Tamburini E, Rosi P, Polati E, Donadello K, Gottin L, Vianello A, Landoni G, Navalesi P, FERS, for the COVID-19 VENETO ICU Network (2021) Outcomes of COVID-19 patients intubated after failure of non-invasive ventilation: a multicenter observational study. Sci Rep 11(1):17730. https://doi.org/10.1038/s41598-021-96762-1 PMID: 34489504; PMCID: PMC8421335
Santamarina MG, Boisier D, Contreras R, Baque M, Volpacchio M, Beddings I (2020) COVID-19: a hypothesis regarding the ventilation-perfusion mismatch. Crit Care 24(1):395. https://doi.org/10.1186/s13054-020-03125-9 PMID: 32631389; PMCID: PMC7338110
Boscolo A, Spiezia L, Correale C, Sella N, Pesenti E, Beghetto L, Campello E, Poletto F, Cerruti L, Cola M, De Cassai A, Pasin L, Eugenio S, Vettor R, Cattelan AM, Simioni P, Navalesi P (2020) Different hypercoagulable profiles in patients with COVID-19 admitted to the internal medicine ward and the intensive care unit. Thromb Haemost 120(10):1474–1477. https://doi.org/10.1055/s-0040-1714350 Epub 2020 Jul 23. PMID: 32702757
Tonetti T, Grasselli G, Rucci P, Alessandri F, Dell'Olio A, Boscolo A, Pasin L, Sella N, Mega C, Melotti RM, Girardis M, Busani S, Bellani G, Foti G, Grieco DL, Scaravilli V, Protti A, Langer T, Mascia L, Pugliese F, Cecconi M, Fumagalli R, Nava S, Antonelli M, Slutsky AS, Navalesi P, Pesenti A, Ranieri VM (2021) Synergistic effect of static compliance and d-dimers to predict outcome of patients with COVID-19-ARDS: a prospective multicenter study. Biomedicines. 9(9):1228. https://doi.org/10.3390/biomedicines9091228 PMID: 34572414; PMCID: PMC8467668
Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, Tubiolo D, Tagliabue P, Zanella A, Grasselli G, Pesenti A (2020) Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med 48(8):1129–1134
Zarantonello F, Andreatta G, Sella N, Navalesi P (2020) Prone position and lung ventilation and perfusion matching in acute respiratory failure due to COVID-19. Am J Respir Crit Care Med 202(2):278–279
Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg AF, De Prost N, Amato M, Carteaux G, Mekontso Dessap A (2020) Effect of positive end-expiratory pressure and proning on ventilation and perfusion in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med 202(12):1713–1717
Safaee Fakhr B, Araujo Morais CC, De Santis Santiago RR, Di Fenza R, Gibson LE, Restrepo PA, Chang MG, Bittner EA, Pinciroli R, Fintelmann FJ, Kacmarek RM, Berra L (2020) Bedside monitoring of lung perfusion by electrical impedance tomography in the time of COVID-19. Br J Anaesth 125(5):e434–e436. https://doi.org/10.1016/j.bja.2020.08.001 Epub 2020 Aug 7. PMID: 32859359; PMCID: PMC7413127
Foronda FAK, Fernandes LR, Lahoz ALC, Johnston C, de Carvalho WB (2021) Electrical impedance tomography clues to detect pulmonary thrombosis in a teenager with COVID-19. Pediatr Radiol:1–4. https://doi.org/10.1007/s00247-021-05199-1 Epub ahead of print. PMID: 34557955; PMCID: PMC8460319
Langer T, Brioni M, Guzzardella A, Carlesso E, Cabrini L, Castelli G, Dalla Corte F, De Robertis E, Favarato M, Forastieri A, Forlini C, Girardis M, Grieco DL, Mirabella L, Noseda V, Previtali P, Protti A, Rona R, Tardini F, Tonetti T, Zannoni F, Antonelli M, Foti G, Ranieri M, Pesenti A, Fumagalli R, Grasselli G (2021) PRONA-COVID Group: Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care 25(1):128
Hedenstierna G, Rothen HU (2012) Respiratory function during anesthesia: effects on gas exchange. Compr Physiol 2:69–96
Grieco DL, Russo A, Romanò B, Anzellotti GM, Ciocchetti P, Torrini F et al (2018) Lung volumes, respiratory mechanics and dynamic strain during general anaesthesia. Br J Anaesth 121:1156–1165
Hedenstierna G, Tokics L, Scaramuzzo G, Rothen HU, Edmark L, Öhrvik J (2019) Oxygenation impairment during anesthesia: influence of age and body weight. Anesthesiology. 131:46–57
Spadaro S, Grasso S, Karbing DS, Fogagnolo A, Contoli M, Bollini G et al (2018) Physiologic evaluation of ventilation perfusion mismatch and respiratory mechanics at different positive end-expiratory pressure in patients undergoing protective one-lung ventilation. Anesthesiology. 128:531–538
Spadaro S, Karbing DS, Mauri T, Marangoni E, Mojoli F, Valpiani G et al (2016) Effect of positive end-expiratory pressure on pulmonary shunt and dynamic compliance during abdominal surgery. Br J Anaesth 116:855–861
Gattinoni L, Quintel M, Marini JJ (2018) Volutrauma and atelectrauma: which is worse? Crit Care 22:264
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F et al (2008) Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355
D’Antini D, Rauseo M, Grasso S, Mirabella L, Camporota L, Cotoia A et al (2018) Physiological effects of the open lung approach during laparoscopic cholecystectomy: focus on driving pressure. Minerva Anestesiol 84:159–167. https://doi.org/10.23736/S0375-9393.17.12042-0
Erlandsson K, Odenstedt H, Lundin S, Stenqvist O (2006) Positive end-expiratory pressure optimization using electric impedance tomography in morbidly obese patients during laparoscopic gastric bypass surgery. Acta Anaesthesiol Scand 50:833–839
Nestler C, Simon P, Petroff D, Hammermüller S, Kamrath D, Wolf S et al (2017) Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 119:1194–1205
Ukere A, März A, Wodack KH, Trepte CJ, Haese A, Waldmann AD, Böhm SH, Reuter DA (2016) Perioperative assessment of regional ventilation during changing body positions and ventilation conditions by electrical impedance tomography. BJA 117(2):228–235. https://doi.org/10.1093/bja/aew188
Spadaro S, Mauri T, Böhm SH, Scaramuzzo G, Turrini C, Waldmann AD et al (2018) Variation of poorly ventilated lung units (silent spaces) measured by electrical impedance tomography to dynamically assess recruitment. Crit Care 22:26
Schaefer MS, Wania V, Bastin B, Schmalz U, Kienbaum P, Beiderlinden M et al (2014) Electrical impedance tomography during major open upper abdominal surgery: a pilot-study. BMC Anesthesiol 14:51
Pereira SM, Tucci MR, Morais CCA, Simões CM, Tonelotto BFF, Pompeo MS et al (2018) Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 129:1070–1081
Spinelli E, Mauri T, Fogagnolo A et al (2019) Electrical impedance tomography in perioperative medicine: careful respiratory monitoring for tailored interventions. BMC Anesthesiol 19:140. https://doi.org/10.1186/s12871-019-0814-7
Steinmann D, Stahl CA, Minner J, Schumann S, Loop T, Kirschbaum A et al (2008) Electrical impedance tomography to confirm correct placement of doublelumen tube: a feasibility study. Br J Anaesth 101:411–418
Rauseo M, Mirabella L, Grasso S et al (2018) Peep titration based on the open lung approach during one lung ventilation in thoracic surgery: a physiological study. BMC Anesthesiol 18:156. https://doi.org/10.1186/s12871-018-0624-3
Zhao Z, Möller K, Steinmann D, Frerichs I, Guttmann J (2009) Evaluation of an electrical impedance tomography-based global inhomogeneity index for pulmonary ventilation distribution. Intensive Care Med 35:1900–1906
Zhao Z, Wang W, Zhang Z, Xu M, Frerichs I, Wu J et al (2018) Influence of tidal volume and positive end-expiratory pressure on ventilation distribution and oxygenation during one-lung ventilation. Physiol Meas 39:034003
Reychler G, Uribe Rodriguez V, Hickmann CE, Tombal B, Laterre P-F, Feyaerts A et al (2018) Incentive spirometry and positive expiratory pressure improve ventilation and recruitment in postoperative recovery: a randomized crossover study. Physiother. Theory Pract:1–7
Karsten J, Heinze H, Meier T (2014) Impact of PEEP during laparoscopic surgery on early postoperative ventilation distribution visualized by electrical impedance tomography. Minerva Anestesiol 80:158–166
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T (2007) Weaning from mechanical ventilation. Eur Respir J 29(5):1033–1056
Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, Navalesi AM, Brozek J, Conti G, Ferrer M, Guntupalli K, Jaber S, Keenan S, Mancebo J, Mehta S, Raoof S (2017) Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 50(2)
Lima JNG, Fontes MS, Szmuszkowicz T, Isola AM, Maciel AT (2019) Electrical impedance tomography monitoring during spontaneous breathing trial: physiological description and potential clinical utility. Acta Anaesthesiol Scand 63(8):1019–1027
Longhini F, Maugeri J, Andreoni C, Ronco C, Bruni A, Garofalo E, Pelaia C, Cavicchi C, Pintaudi S, Navalesi P (2019) Electrical impedance tomography during spontaneous breathing trials and after extubation in critically ill patients at high risk for extubation failure: a multicenter observational study. Ann Intensive Care 9(1):88
Bickenbach J, Czaplik M, Polier M, Marx G, Marx N, Dreher M (2017) Electrical impedance tomography for predicting failure of spontaneous breathing trials in patients with prolonged weaning. Crit Care 21(1):177
Moon DS, Huh JW, Hong SB, Koh Y, Lim CM (2021) Dynamic inhomogeneity of aeration along the vertical axis of the lung may predict weaning failure regardless of diaphragm dysfunction. J Crit Care 65:186–191
Wang G, Zhang L, Li B, Niu B, Jiang J, Li D, Yue Z, Weng Y (2021) The application of electrical impedance tomography during the ventilator weaning process. Int J Gen Med 14:6875–6883
Zhao Z, Peng SY, Chang MY, Hsu YL, Frerichs I, Chang HT, Moller K (2017) Spontaneous breathing trials after prolonged mechanical ventilation monitored by electrical impedance tomography: an observational study. Acta Anaesthesiol Scand 61(9):1166–1175
Longhini F, Bruni A, Garofalo E et al (2020) Chest physiotherapy improves lung aeration in hypersecretive critically ill patients: a pilot randomized physiological study. Crit Care 24:479. https://doi.org/10.1186/s13054-020-03198-6
Corley A, Spooner AJ, Barnett AG et al (2012) End-expiratory lung volume recovers more slowly after closed endotracheal suctioning than after open suctioning: a randomized crossover study. J Crit Care 27(742):e1–e7
Wolf GK, Grychtol B, Frerichs I et al (2007) Regional lung volume changes in children with acute respiratory distress syndrome during a derecruitment maneuver. Crit Care Med 35:1972–1978
Putensen C, Hentze B, Muenster S, Muders T (2019) Electrical impedance tomography for cardio-pulmonary monitoring. J Clin Med 8(8):1176. Published 2019 Aug 7. https://doi.org/10.3390/jcm8081176
Brochard L, Slutsky A, Pesenti A (2017) Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 195:438–442
Grieco DL, Menga LS, Eleuteri D, Antonelli M (2019) Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol 85:1014–1023. https://doi.org/10.23736/S0375-9393.19.13418-9
Yoshida T, Grieco DL, Brochard L, Fujino Y (2020) Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr Opin Crit Care 26:59–65. https://doi.org/10.1097/MCC.0000000000000691
Bordes J, Goutorbe P, Cungi PJ et al (2016) Noninvasive ventilation during spontaneous breathing anesthesia: an observational study using electrical impedance tomography. J Clin Anesth 34:420–426
Corley A, Caruana LR, Barnett AG et al (2011) Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 107:998–1004
Riera J, Pérez P, Cortés J et al (2013) Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 58:589–596
Borges JB, Suarez-Sipmann F, Bohm SH, Tusman G, Melo A, Maripuu E, Sandstrom M, Park M, Costa EL, Hedenstierna G, Amato M (2012) Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J Appl Physiol (1985) 112:225–236
Bluth T, Kiss T, Kircher M, Braune A, Bozsak C, Huhle R, Scharffenberg M, Herzog M, Roegner J, Herzog P, Vivona L, Millone M, Dossel O, Andreeff M, Koch T, Kotzerke J, Stender B, Gama de Abreu M (2019) Measurement of relative lung perfusion with electrical impedance and positron emission tomography: an experimental comparative study in pigs. Br J Anaesth 123:246–254
Acknowledgements
We thank Draeger and SIAARTI for the opportunity to participate and share knowledge about the EIT.
Funding
Thanks to the unconditional contribution of Draeger.
Author information
Authors and Affiliations
Consortia
Contributions
MR, ES, TM, GC, and PN conceived the idea of this document and wrote the first draft, together with the support of SS, NS, FL, and DS, who revised and contributed to the manuscript. AG supervised the project. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rauseo, M., Spinelli, E., Sella, N. et al. Expert opinion document: “Electrical impedance tomography: applications from the intensive care unit and beyond”. J Anesth Analg Crit Care 2, 28 (2022). https://doi.org/10.1186/s44158-022-00055-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44158-022-00055-6