Abstract
This article describes the discontinuous adsorption of Mn(II) on kaolin from Guelma, Algeria (KGA), and blast furnace slag from Sider, Algeria (BFS), under the effect of various parameters, namely the contact time, the stirring speed, the pH of the medium, the solution temperature, the adsorbent dosage, the solid particle size, and the initial concentration. Also studied were the models of adsorption, namely the isotherms of adsorption, the kinetics of adsorption, and the thermodynamic study of Mn on the examined adsorbents. Characterization tests have indicated that kaolin consists essentially of hydrated aluminum silicate. The BFS is mainly composed of silicates, aluminates, lime, and magnesium oxide. The specific surface areas of kaolin and BFS calculated using the BET were defined at 134.2 and 238.6m2/g. The adsorption rate of Mn(II) on KGA and BFS is better after 50 and 60 min of contact at Vag: 150 rpm; pH: 5.2; Øs: 100 μm; T: 20 °C; Ms: 1 g, respectively. Maximum adsorption capacities are 36.76 mg/g (KGA) and 59.88 mg/g (BFS). Examination of the adsorption isotherms revealed that the Langmuir model is more appropriate to the experimental data (R2 = 0.99). The values of the Freundlich (n), Langmuir (RL), and Temkin (bt) parameters indicate that the adsorption is favorable. The kinetic examination demonstrated that the pseudo-second-order kinetic model is more adopted for the adsorption of Mn(II) on KGA and BFS (R2 = 0.99). Furthermore, the transfer of Mn(II) from the solution to the surfaces of the investigated adsorbents is controlled by external and internal diffusion. The thermodynamic study brought to light that the adsorption processes carried out were spontaneous, exothermic, and less entropic. This work showed that KGA and BFS can be used as low-cost adsorbents for the removal of Mn(II) ions in aqueous media, and BFS has higher affinities for manganese ion adsorption.
Similar content being viewed by others
Introduction
In recent years, water pollution has begun to increase and become threatening. This danger is mainly caused by the extension of town planning and the evolution of industries [1]. This problem has become a universal concern because it can cause disastrous consequences for people and their environments. Currently, the most significant pollution is caused by metal ions. Indeed, metals pose a constant risk to man and his environment due to their toxicity and accumulation.
In this regard, new techniques have been directed towards the protection of man and his environment, such as chemical precipitation, membrane techniques, ion exchange, coagulation-flocculation, and adsorption [2]. In this study, we chose the elimination of manganese by adsorption because it is efficient, simple, and less expensive. The experimental approach in this work consisted of eliminating manganese dissolved in water by adsorption phenomenon using blast furnace slag and kaolin, separately, as adsorbents. The objectives of this manipulation are the elimination of manganese ions in solution and the recovery of blast furnace slag and kaolin as adsorbents on the one hand. On the other hand, the comparison between their adsorbent powers.
Kaolin is a clay mineral generally composed of kaolinite and quartz. Its shape is divided into dioctahedral and trioctahedral minerals. It is used in various industrial applications, namely ceramics, paints, rubbers, plastics, cosmetics, and medicines. For some years, and given the high cost of adsorbents, it has been oriented in the field of water treatment and especially in the adsorption process. Indeed, based on studies conducted, it was reported that kaolin is used in processes for the removal of metal ions.
In this approach, Chouchane et al. [2] showed that nickel adsorption on kaolin in solution is favorable, where the capacity and rate of adsorption are respectively 45.39 mg g-1 and 89.3% after 60 min of agitation. According to Ibrahim et al. [3], zeolite prepared from Egyptian kaolin can be used as an inexpensive and effective material for metal cation removal, specifically Cu(II), Cd(II), Cu(II), Ni(II), and Zn(II). Chouchane et al. [4] reported that the adsorption of copper on kaolin is thermodynamically feasible, spontaneous, exothermic, and less entropic. Within the same context, Mustafa et al. [5] clarified that the sorption of Cr, Cd, and Zn reached equilibrium in a relatively fast time (15 min), and the adsorption is more efficient at pH 5.85. However, Dawodu et al. [6] reported that unmodified Nigerian kaolin has a high affinity for Mn(II) and Ni(II) adsorption, and the highest elimination is at pH 6.
Blast furnace slag is a co-product of the steel industry. It is produced during the production of cast iron in the blast furnace from iron ores. It consists essentially of lime, silica, alumina, and magnesium oxide [7]. According to the literature, the annual production of steel slag is about 100 million tons [7], part of which is used in road shaping and building materials. The rest is gathered in the open air, which generates not only an immense monetary loss but also a threat to man and his environment. It is in this context that research has been directed in order to enhance this co-product and protect the environment from harmful inputs.
Indeed, according to the research work carried out, blast furnace slag has shown a good affinity for the metal ions’ adsorption in solution. El-Dars et al. [8] cited that the adsorption of copper on water-cooled blast furnace slag in batch mode is more efficient for particles with a diameter of 0.3 mm. Chouchane et al. [7] have shown that the adsorption of nickel on blast furnace slag is feasible, and its efficiency depends on the determining parameters used. According to Wang et al. [9], steel slag is an effective adsorbent in flotation wastewater containing Cu(II) and Pb(II). Ngoc Lee et al. [10] indicated that slag oxalate derived from blast furnace slag is considered an adsorbent of choice for the maximum removal of cobalt from the solution.
Manganese is an essential element for human survival, but in high concentrations, it causes health problems. Indeed, manganese acts mainly at the level of the respiratory system and the brain; it causes neurological and behavioral effects [11, 12]. Therefore, it is important to minimize the concentration of manganese in the water.
Clays [13], activated carbon [14], sawdust [15], and composites have all been used in the literature to remove M(II) from solutions. According to the same scientific resources, these adsorbents showed good adsorption capacity.
Analyzes by physico-chemical and electronic characterization techniques, namely FRX, DRX, and SEM, were used to determine the composition of the solids exploited. The effects of the determining factors, such as the contact time (teq, min), speed of agitation (Vag, rpm), pH, temperature (T, °C), and initial concentration (C0, mg/L), were evoked in order to measure the maximum capacity of adsorption. The adsorption isotherms of Freundlich, Langmuir, and Temkin were applied to infer the interaction between manganese and exploited adsorbents. Adsorption kinetics have been examined by relevant models, including Lagergren, Blanchard, and Weber Morris. The nature of the adsorption processes was determined by a thermodynamic study.
In the near future, it would be desirable to work in dynamic mode with real solutions inspired by recent work in this field. It is important to perform simulations based on high-value mathematical models as presented by Nadeem et al. [16], Amjad et al. [17], and Nadeem et al. [18].
Methods/experiment
Materials
The white-colored kaolin was recovered from the clay deposit in Guelma, Algeria. The gray-colored blast furnace slag was collected from the El-Hadjar steel complex in Annaba, Algeria.
Adsorbent preparation
The kaolin under consideration (KGA) was prepared and treated using the experimental approach presented by Chouchane et al. [2]. This experimental protocol is presented as follows:
-
Grinding of raw kaolin to particles smaller than 1 mm
-
Crushed kaolin washing followed by drying in the open air for 72 h
-
Purification and oxidation of the organic materials [19]
-
Washing and dispersion of the kaolin [5]
-
Activation of kaolin by hydrochloric acid [20]
-
Sieving at different diameters
-
Preservation of samples in desiccators
The slag from the blast furnace used was treated according to the protocol defined by Chouchane et al. [7]. This experimental approach is presented as follows:
-
Washing with distilled water and air drying for 48 h
-
Grinding to fine particles
-
Sieving at different diameters (200, 300, 400, and 500 μm)
-
The samples with different grain sizes were separated, washed with distilled water, steamed at 105 °C, and stored in plastic boxes
Analytic methods
Mn(II) concentration was measured by atomic absorption spectroscopy (PerkinElmer 3110) The pH of the solution was measured with a pH metter (Ericsson). The characterization was carried out by X-ray fluorescence (Siemens SRS 3000) and X-ray diffraction (Rigaku Ultim IV), and the morphology was examined by a scanning electron microscope (Zeiss EVO MA25).
Adsorption protocol
A series of discontinuous experiments were carried out to investigate the sorption of Mn(II) on the adsorbents under consideration, namely KGA and BFS. The experimental approach is summarized by the addition, in a 1-l beaker, of 1 g of adsorbent to solutions prepared with manganese nitrate (Mn(NO3)2, 4H2O). The continuous mixing of the solution was ensured during all the tests by a mechanical stirrer operating at different speeds. The temperature was controlled with a water bath equipped with a thermostat. Adsorption kinetics were followed by a sampling of 5 ml every 10 min. Chouchane et al. provided this experimental protocol [2, 4, 7].
Equation 1 was used to calculate the adsorption capacity of Mn(II):
The adsorption efficiency of Mn(II) was calculated using Eq. 2:
where C0 is the initial concentration of the metal solution (mg/L), Ct is the concentration of the metal solution after a time t (mg/L), Ce is the concentration of the metal solution after at equilibrium (mg/L), V is the volume of the solution (L), and m is the adsorbent mass (g).
The achieved experimental conditions are summarized as follows:
-
Contact effect: C0: 30 mg/L; Vag.: 50 rpm; pH: 5.2; T = 20 °C; Øs: 150 μm; Ms: 1 g
-
Effect of adsorbent: C0: 30 mg/L; Vag.: 50 rpm; pH: 5.2; T = 20 °C; Øs: 150 μm; Ms: 0.4, 0.6, 0.8, 1., 1.2, 1.4 g
-
Effect of agitation speed: C0: 30 mg/L; Vag: 50, 100, 150, and 200 rpm; pH: 5.2; T = 20 °C; Øs: 150 μm; Ms: 1 g
-
Effect of pH: C0: 30 mg/L; Vag.: 150 rpm; pH = 2.8, 4.4, 5.2, and 6; T: 20 °C; Øs: 150 μm; Ms: 1 g
-
Effect of T: C0: 30 mg/L; Vag: 150 rpm; pH: 5.2; T: 18, 40, and 50 °C; Ø:150 μm; Ms: 1 g
-
Effect of Øs: C0: 30 mg/L; Vag.: 150 rpm; pH: 5.2; T = 20 °C; Øs: 50, 100, 150, and 200 μm; Ms: 1 g
-
Effect of C0: C0: 10–100 mg/L; Vag: 150 rpm; pH: 5.2; Øs: 100 μm; T: 20 °C; Ms: 1 g
where BFS is the blast furnace slag, C0 is the initial concentration (mg/L), Vag is the agitation speed (rpm), Øs is the adsorbent particle size (μm), Ms is the adsorbent mass (g), T is the solution temperature (°C).
Results and discussion
Characterization of KGA and BFS
Physico-chemical tests conducted by XRF pointed out that the kaolin of Guelma, Algeria (KGA), is composed mostly of silica (46.58%), alumina (36.22%), and a minimal percentage of oxides (Table 1) [2]. These results have been justified by the investigations carried out using XRD in Fig. 1a.
a Diffractogram of KGA sample, b and c SEM images of KGA [2]
From Fig. 2a, it has been noticed that a smooth surface is formed of multiple sheets stacked in the form of agglomerates [2, 21]. On the other hand, in Fig. 2b, a more porous structure was observed with the appearance of new sites, which led to an increase in the specific surface area [2, 22]. The calculated specific surface area is 134.2 m2/g.
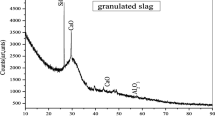
a Diffractogram of BFS sample [7], b EDX results, c and d SEM images of BFS
According to the results provided by X-ray fluorescence spectrometry (XRF), the blast furnace slag (BFS) is globally composed of alkali oxides and acidic oxides. The percentage rates of the major elements are 38.31% CaO, 14.12% Al2O3, 5.54% MgO, and 36.52% SiO2 (Table 1). The tests performed by X-ray diffraction (XRD) confirmed the results obtained by XFR. Indeed, we found a dominance of CaO, MgO, Al2O3, and SiO2 (Fig. 2a). It should be noted that the tests carried out by SEM-XDE also specified that the blast furnace slag (BFS) is made up particularly of SiO2, CaO, Al2O3, and MgO (Fig. 2b). From Fig. 2c, it was observed that the BFS grains have a conchoidal shape, a wide particle size distribution, and smooth surfaces. On the other hand, in Fig. 2d, only visible grain edges and no visible porous structures were observed.
The specific surface areas of the BFS particles were obtained by using the nitrogen gas adsorption-desorption method. The isotherm data for nitrogen gas desorption at 77 k were analyzed with the Brunauer, Emmett, and Teller model (model BET). The investigations revealed that the specific surface is 238.6m2/g. In this work.
Effect of contact time
The study of the effect of the contact time on the adsorption process is essential because it makes it possible to determine the equilibrium time and thus reduce the duration of the experiment. With this in mind, we separately investigated the effect of contact time on Mn(II) sorption on KGA and BFS under the aforementioned experimental conditions.
Figure 3a depicts the effect of contact time on Mn(II) adsorption in solution on KGA and BFS. According to Fig. 3a, a significant increase in adsorption rate was observed beforehand, followed by a slight increase, and ended with a constant value for all processes performed. The times shown for the KGA and BFS are 50 and 60 min, respectively. The rapid adsorption is mainly elicited by the availability of free adsorption sites on the surface of the adsorbents under investigation. On the other hand, the slow adsorption is mainly caused by the reduction of the active sites. The rapid process is controlled by external diffusion, which is caused by the mass transport of Mn(II) ions from the solution to the surface of the adsorbent under study [6, 7, 23].
It should be noted that manganese adsorption by Nigerian kaolin occurs after 180 min [6]. Adsorption of Mn(II) ions on electric arc furnace steel slag, on the other hand, took 60 min [24]. This effect indicates that Mn(II) ion adsorption on KGA and BFS is relatively fast.
Effect of adsorbent dosage
The effect of adsorbent dose is an essential parameter of adsorption processes. In this regard, we looked into the effect of adsorbent mass on Mn(II) adsorption in solution. Figure 3b depicts the effect of the adsorbent dose on Mn(II) adsorption on KGA and BFS.
The kinetic study revealed that the rate of Mn(II) adsorption increases with the dose of the adsorbents studied (Fig. 3b). The increase in the rate of adsorption is elicited mainly by the multiplication of active adsorption sites [24,25,26]. From Fig. 3b, a slight decrease in adsorption capacity was also observed at m > 1 g. This consequence is prompted by the emergence of the coalescence phenomenon [26, 27]. From this result, we estimated that the optimal mass corresponds to 1 g for the different adsorbents used.
Effect of agitation speed
The agitation speed is a very influential parameter in adsorption processes since it avoids the decantation of the adsorbent and promotes the transfer of manganese metal ions from the solution to the surface of the adsorbent, forming the outer boundary layer [4]. In this context, we varied the stirring speed from 100 to 300 rpm (supp. Figure 1).
The adsorption kinetics under the aforementioned conditions revealed that eliminating Mn(II) on KGA and BFS at a speed of 150 rpm is more efficient (Fig. 4a). The increase in adsorption capacity with the increase in stirring speed from 50 to 150 rpm is due to the good distribution of the solid in the solution [4, 28]. At 200 rpm, the kinetic study revealed that the values of the adsorption capacities vary abnormally with the contact time (Fig. 4). This disorder is surely caused by the desorption of metal ions from the adsorbent surfaces at high agitation speeds (Fig. 4a) [29].
The effect of pH on adsorption
According to subsequent studies, the pH of the medium has an extraordinary effect on the adsorption processes [2, 4, 6, 10]. Indeed, the pH influences the adsorbent’s surface charge, and it also has a direct impact on the metal ions in the solution [6]. In this study, we worked with various pH values, namely 2.2, 4.4, 5.2, and 6 under the experimental conditions mentioned above.
Supplementary Fig. 2 depicts the effect of pH on Mn(II) adsorption by KGA and BFS. The kinetic analysis revealed that Mn(II) ion adsorption on the adsorbents under consideration improves at pH 5.2 (Fig. 4b). The adsorption rates of Mn(II) ions on KGA and BFS were found to be 78.63% and 87.2%, respectively.
Adsorption kinetics at a strongly acidic pH (2.2) revealed that the elimination of Mn(II) ions is unfavorable. Indeed, the abundant presence of H+ creates a proton cloud, which inhibits the adsorption of Mn(II) ions on the surfaces of KGA and BFS [30, 31]. At pH 4, Mn(II) adsorption processes on KGA and BFS improved significantly. In fact, with the rise in the initial pH of the solution, the competition for the active sites diminishes and the electrical repulsion forces weaken, which facilitates the transfer of metal ions from the solution to the adsorbent (increasing the process of adsorption) [32]. Furthermore, at pH 6, the adsorption rate decreased, which can be attributed to the precipitation of Mn(II) ions in the form of Mn(OH)2. Based on these findings, it was determined that the optimal pH for Mn(II) ion adsorption on the investigated adsorbents was 5.2.
The effect of temperature
Solution temperature is a determining variable in adsorption processes since it influences the adsorbent-adsorbent interface and the movement of pollutants [2, 27, 33]. In this approach, we varied the temperature of the medium from 20 to 60 °C (supp. Figure 3).
According to this illustration, the adsorption of Mn(II) ions in the two processes investigated is inversely proportional to the temperature of the solution (Fig. 5a). This effect is caused certainly by a decrease in kinetic energy in Mn(II) ions upon temperature rise. Indeed, as the temperature increases, the kinetic energy gradually decreases, which negatively influences the frequency generated between the adsorbate and the adsorbent [7, 34]. This section predicts that the adsorption of Mn(II) on kaolin and BFS was favorable at low adsorption temperatures and was exothermic nature [2, 4].
Effect of particle size
The adsorption kinetics of Mn(II) ions on kaolin and BFS as a function of adsorbent particle size is depicted in supp. Figure 4.
The experimental data revealed that the highest adsorption rate was indicated for particles with a size equal to 100 μm. In the same context, it was observed that the adsorption capacity gradually decreased as the particle size intensified (Fig. 5b). This effect is probably caused by the increase in the surface area of the solids, which regenerates new active adsorption sites [6, 35]. For particles with a size of 50 μm, modest adsorption of Mn(II) ions on kaolin and BFS was observed. This deceleration is possibly caused by the rallying of the particles of the adsorbent and thus the return to larger sizes (coalescence phenomenon) [7, 36].
Effect of initial concentration
The effect of the initial concentration of Mn(II) ions on the maximum adsorption capacity per gram of kaolin and BFS was investigated (Fig. 6). The experiment was conducted under optimal conditions using initial concentrations between 10 and 100 mg/L.
According to Fig. 6a, it was indicated that the adsorption capacity of kaolin and BFS is proportional to the initial concentration of manganese between 10–70 mg/l and 10–80 mg/l, respectively. Furthermore, the adsorption capacities become constant with these values (kaolin: 44.58 mg/g, BFS: 59.88 mg/g). The increase in adsorption capacity is due to the high initial concentration, which acts as a driving force to promote mass transfer [37]. The presence of the horizontal plateau results from the unavailability of the active adsorption sites, namely the saturation of the adsorbents studied [38].
In both processes, the sorption rate regressed with the multiplication of Mn(II) ions, as shown in Fig. 7b. The decreasing rate of adsorption of Mn(II) ions on the investigated adsorbents was caused by an imbalance between free adsorption sites and the increasing number of available ions [2, 39].
According to the experimental data, the adsorption of manganese on kaolin and on BFS in solution is influenced in the same way by the variation of pH, agitation, temperature, and particle size (Figs. 4 and 5). However, it is different under the effect of the contact time and the initial concentration (Figs. 2 and 6).
From this step, we conclude that manganese adsorption is better on BFS than on KGA. According to the bibliography, the KGA and BFS used in this study can be considered adsorbents of choice for the elimination of Mn(II) ions in solution (Table 2).
Isothermal adsorption models
The adsorption isotherm is the illustration of the interactions existing between the adsorbent and the adsorbate. In this approach, we exploited appropriate mathematical models, namely the Freundlich, Langmuir, and Temkin models. Their linear form is represented by Eqs. 3, 4, and 5, respectively. The one-dimensional separation factor (RL) parameter of the Langmuir model is given by Eq. 6.
The linear form of their equation is represented by Eqs. 3, 4 and 5, successively [2, 45].
Its mathematical form is given as follows [6, 15]:
where qe is the capacity adsorbed at equilibrium (mg g−1), Ce is the concentration at equilibrium (mg L−1), qmax is the maximum capacity adsorbed (mg g−1), KF is the Freundlich isotherm constant, 1/n is the empirical parameter Freundlich, b is the thermodynamic constant of the adsorption equilibrium (L.mg−1), AT is the Temkin isotherm equilibrium binding constant (L/g), bT is the Temkin isotherm constant kJ mol−1), R is the universal gas constant, T is temperature at 298 K, and BT is the constant related to heat of sorption.
Adsorption isotherms of the examined models, namely Freundlich, Langmuir, and Temkin, to the experimental data are shown in supp. Figures 5, 6, and 7. The results obtained are shown in Table 3.
Based on these findings, the Langmuir model was determined to be the best fit for the experimental data from the processes of adsorption of Mn(II) ions on KGA and BFS, respectively (Table 3). Indeed, the correlation coefficients of the Langmuir model resulting from the two processes studied are greater than those resulting from the Freundlich and Temkin models. In addition, the calculated maximum adsorbed capacities of Mn (II) ions on KGA (37.09 mg/g) and BFS (60.31 mg/g) are very close to the maximum experimental capacities, namely 36.76 mg/g and 59.88 mg/g, respectively (Table 3).
From Fig. 7a and b, we noticed a good adequacy between the experimental data and the theoretical data from the Langmuir model, which further demonstrates that the processes carried out adhere more to the Langmuir model. The Freundlich parameter (n) for both processes is between 1 and 10 (Table 3), indicating that Mn(II) ion adsorption on KGA and BFS is favorable [2, 15].
According to the Langmuir adsorption isotherms (Fig. 7a and b), at low concentrations, strong sorption was observed, followed by slow adsorption, and finally stability (adsorbent surface saturation), resulting in the formation of a horizontal line. These adsorption isotherms are typical of single-layer adsorptions presented by Langmuir’s hypothesis. This observation confirms that Mn(II) ion adsorption on KGA and BFS occurred on a uniform monolayer surface [46].
The Freundlich parameter (n) for both processes is between 1 and 10 (Table 3), indicating that Mn(II) ion adsorption on kaolin and BFS is favorable [15]. In Fig. 6c, the separation coefficient (RL) ranges from 0 to 1, indicating that Mn(II) ion adsorption on KGA and BFS is favorable [2, 7].
According to the documentation, it has been noted that the adsorption of Mn(II) on sawdust was carried out on a heterogeneous surface [15]. On the other hand, the adsorption of Mn(II) on charcoal was carried out on a homogeneous surface [14]. It was also indicated that the Mn2 + adsorption on sand coated with Fe and Mn oxide was carried out on a homogeneous and heterogeneous surface [41]. These data allowed us to deduce that the mechanism of adsorption depends on the quality of the adsorbent and the adsorbate [41].
Kinetic studies
Pseudo-first-order and pseudo-second-order models were used to control the adsorption rate of Mn(II) ions on KGA and BFS. On the other hand, appropriate models were used to investigate the external and internal diffusion of Mn(II) ions from the solution to the surfaces of the adsorbents under consideration.
The pseudo-first-order and pseudo-second-order models are represented by Eqs. 7 and 8, respectively [14, 15].
where qe is the adsorbed quantity at equilibrium (mg/g), q is the amount of metal ions adsorbed at time t (mg/g), t is the time of adsorption process (min), kL is the constant pseudo-first order kinetic equation (min−1), and kb is the constant of pseudo-second order speed equation (min−1).
The plots of the functions ln (qe-q) = f(t) and t/q = f(t) are represented in Fig. 8. Kinetic parameters are shown in Table 4.
From Table 4, we noted that the value of the correlation coefficient (R2) of the processes executed for the pseudo-second-order is higher than that of the pseudo-first-order. In addition, the adsorbed quantities from the pseudo-second-order model are close to the experimental capacities. This result allowed us to deduce that the adsorption of manganese in solution on the KGA and on the BFS follows pseudo-second-order kinetics [2, 6, 14, 47].
Appropriate models, namely the models of external diffusion [2, 48] and intraparticle diffusion [7, 49], were used to identify the rate-limiting steps controlling the adsorption rate of Mn(II) ions on KGA and BFS. These models are represented by Eqs. 9 and 10, respectively.
where q is the amount of metal ions adsorbed at time t (mg/g), Ct is the remaining concentration at time t (mg/L), t is the time of adsorption process, kint is the diffusion rate constant in the pores (mg/m. min ½), kext is the external diffusion constant (mg.L−1.min−1), and C is the intercept and it’s tied to the boundary layer.
The graphs resulting from the functions of ln(Ct) = f(t) and q = f(t1/2) for the sorption of manganese on KGA and BFS are illustrated in Fig. 9a and b, respectively. The kinetic parameters are displayed in Table 5.
From Fig. 9b, it was shown that the traces representing the adsorption of manganese on KGA and BFS did not pass through the origin, i.e., the interception, and are different from zero. In addition, the shape of the plots is multilinear, and the correlation coefficients (R2) were above 0.90 (Table 4), which points out that internal diffusion is not the only mechanism controlling the Mn(II) ions adsorption process on the KGA and the BFS [49, 50].
According to the values of the intercept (Cint) (Table 5), it was noted that the adsorption of Mn(II) on KGA was limited by a higher thickness [6].
In Fig. 9a, the plots of the considered processes are linear, and according to the values displayed in Table 5, the coefficients of the corresponding regressions exceed 0.97. This outcome tells us that the Mn(II) ion transport from the solution to the adsorbent surfaces of KGA and BFS was also controlled by external diffusion [2, 4, 50].
As a result, it was concluded that the adsorption of Mn(II) ions on the investigated adsorbents is governed by external and internal diffusion [51]. This outcome has also been indicated in the processes of nickel adsorption on kaolin [2] and on the slag of the blast furnace [7].
To better understand the adsorption processes investigated and to provide more clarity on the limiting steps controlling the Mn(II) ions adsorption on the adsorbents studied, we studied the appropriate equation for external diffusion (Eq. 9).
Thermodynamics study
The specification of the nature of the processes studied involves the determination of the thermodynamic parameters, namely ΔG°, ΔH°, and ΔS° at different temperatures. Equation 11 is used to calculate the Gibbs free energy (ΔG°). The enthalpy (ΔH°) and entropy (ΔS°) are obtained using Eq. 12.
The distribution coefficient (kd) is given by Eq. 13 [2, 6].
The plots resulting from the equation ln(kd) = f(1/T) are represented in Fig. 9c. The values of the thermodynamic parameters as well as the values of the distribution coefficients at different temperatures are displayed in Tables 6 and 7.
From Fig. 4, we observed a good correlation, which means that the theoretical values are close to the experimental values. From the slope and the intercept, we calculated the free enthalpy and the entropy, respectively.
From Tables 6 and 7, it has been indicated that the values of ΔG° are negative and increase in absolute value with the rise in temperature. This identification tells us that the processes studied are spontaneous, and their spontaneity increases with the heating of the solution [52, 53]. The negative enthalpy values indicated that the processes involved were exothermic [2, 4, 7], and Mn(II) ions were physically absorbed on the investigated adsorbents (2 < ΔH < 21 kJ/mol) [6, 54]. Negative entropy values (ΔS°) indicated that randomness was reduced at the adsorbent/adsorbate interface during Mn(II) ion adsorption processes on kaolin and BFS [2, 4, 7].
It is important to mention that the same nature of these processes was observed during the process of elimination of Ni(II), Cu(II), and Zn(II) on kaolin [2, 4, 55] and also during the elimination of Ni(II), Pb(II), and Cr(III) on blast furnace slag [6, 56, 57].
Conclusions
The slag from the blast furnace of the El Hadjar iron and steel complex and Guelma kaolin were used as adsorbents for the suppression of Mn(II) ions in solution via discontinuous adsorption. Characterization tests indicated that kaolin consists mainly of silica (46.58%) and alumina (36.82%). On the other hand, BFS is essentially composed of silica (32.52%), lime (38.31%), and alumina (14.12%). The specific surface areas calculated are 134.2 and 238.6m2/g, respectively, for kaolin and BFS. The kinetic study mentioned that the adsorption processes considered are influenced by various parameters, namely contact time, adsorbent dose, agitation of the medium, pH of the solution, temperature of the solution, particle size of the solid, and initial concentration of the solute. According to the experimental data, the maximum adsorbed amount of Mn(II) on kaolin and BFS was 36.76 mg/g and 59.88 mg/g, respectively, under optimal experimental conditions (C0 for kaolin: 70 mg/L, C0 for BFS: 80 mg/L, Vag: 150 rpm; pH: 5.2; Øs: 100 μm; T: 20 °C; Ms: 1 g). From the same source, Mn(II) ion adsorption on kaolin and BFS reached equilibrium after 50 and 60 min of contact time, respectively. The Langmuir adsorption isotherm model has been more successfully applied to experimental equilibrium data (R2BFS = 0.99, R2KGA = 0.99) than the Freundlich (R2BFS = 0.86, R2KGA = 0.92), and Temkin (R2BFS = 0.92, R2KGA = 0.93) adsorption isotherms in the processes examined. According to the kinetic parameters, the elimination of Mn(II) ions by kaolin and BFS follows pseudo-second-order kinetics, where the correlation coefficients are equal to 0.99. The experimental results also revealed that external diffusion and intraparticle diffusion control the transfer of Mn(II) ions from the solution to the adsorbent surfaces of the KGA and the BFS. The negative values of the thermodynamic parameters ΔG°, ΔH°, and ΔS° insinuate that the examined processes are spontaneous, exothermic, and less entropic. The values of ΔH° clarified that the removal of manganese by the adsorbents studied is a physical adsorption. From these results, it was shown that the solids examined can be used as economical, safe, and efficient adsorbents for the removal of manganese ions from wastewater. It was also revealed that the adsorption capacity of BFS is greater than that of KGA under our experimental conditions.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- BFS:
-
The blast furnace slag
- KGA:
-
Kaolin from guelma, algeria
- CRTI:
-
Research center in industrial technologies
- URMM:
-
Research unit in mining and metallurgy
References
Pakulski D, Czepa W, Witomska S, Aliprandi A, Pawluc P, Patroniak V, Ciesiel-ski A, Samorì P (2018) Graphene oxide-branched polyethylenimine foams for efficient removal of toxic cations from water. J Mater Chem A 6:9384–9390. https://doi.org/10.1039/C8TA01622D
Chouchane T, Boukari A (2022) Impact of influencing parameters on the adsorption of nickel by kaolin in an aqueous medium. Anal Bioanal Chem Res. 9(4):381–399. https://doi.org/10.22036/ABCR.2022.325691.1716
Ibrahim HS, Jamil TS, Hegazy EZ (2010) Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II Isotherm models. J Hazard Mater 182:842–847. https://doi.org/10.1016/j.jhazmat.2010.06.118
Chouchane T, Yahi M, Boukari A, Balaska A, Chouchane S (2016) Adsorption of the copper in solution by the kaolin. J Mater Environ Sci 7(8):2825–2842
Mustapha S, Ndamitso MM, Abdulkareem AS, Tijani JO, Mohammed AK, Shuaib D (2019) Potential of using kaolin as a natural adsorbent for the removal of pollutants from tannery wastewater. Heliyon 5:e02923. https://doi.org/10.1016/j.heliyon.2019.e02923
Dawodu FA, Akpomie KG (2014) Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite clay. J Mater Res Technol 3(2):129–141. https://doi.org/10.1016/j.jmrt.2014.03.002
Chouchane T, Khireddine O, Boukari A (2021) Kinetic studies of Ni(II) ions adsorption from aqueous solutions using the blast furnace slag (BF slag). J Eng Appl Sci 68:00039–00043. https://doi.org/10.1186/s44147-021-00039-3
El-Dars Farida MSE, Elngar Marwa AG, Abdel-Rahim STh, El-Hussiny NA, Shalabi MEH (2015) Kinetic of nickel (II) removal from aqueous solution using different particle size of water-cooled blast furnace slag. Desalination Water Treat 54:769–778. https://doi.org/10.1080/19443994.2014.883578
Wang L, Fu P, Ma Y, Zhang X, Zhang Y, Yang X (2022) Steel slag as a cost-effective adsorbent for synergic removal of collectors, Cu(II) and Pb(II) ions from flotation wastewaters. Miner Eng 183:107593. https://doi.org/10.1016/j.mineng.2022.107593
Le Ngoc QT, Vivas EL, Cho K (2021) Oxalated blast-furnace slag for the removal of Cobalt(II) ions from aqueous solutions. J Ind Eng Chem 95:57–65. https://doi.org/10.1016/j.jiec.2020.12.003
Sheng X, Zhaohui Z, Zhihui W (2022) Potentially toxic elements have adverse effects on moss communities in the manganese mines of Southern China. Environ Pollut 305:119255. https://doi.org/10.1016/j.envpol.2022.119255
Lindner S, Lucchini R, Broberg K (2022) Genetics and epigenetics of manganese toxicity. Curr Environ Health Rpt 9(4):697–713. https://doi.org/10.1007/s40572-022-00384-2
AlTowyan L, AlSagabi S, AlAjyan T, AlSulami K, Goumri-Said S (2022) The removal of manganese ions from industrial wastewater using local Saudi and commercial bentonite clays. Groundw Sustain Dev 19:100821. https://doi.org/10.1016/j.gsd.2022.100821
Meshram YK, Gunjate JK, Khope RU (2020) Studies on adsorption characteristics of manganese onto coal based chemically modified activated carbon. Mater Today Proc 29:1185–1191. https://doi.org/10.1016/j.matpr.2020.05.428
Qomi MH, Eisazadeh H, Hosseini M, Namaghi HA (2014) Manganese removal from aqueous media using polyaniline nanocomposite coated on wood sawdust. Synth Met 194:153–159. https://doi.org/10.1016/j.synthmet.2014.04.016
Nadeem S, Khan MN, Abbas N (2020) Transportation of slip effects on nanomaterial micropolar fluid flow over exponentially stretching. Alex Eng J 59(5):3443–3450. https://doi.org/10.1016/j.aej.2020.05.024
Amjad M, Zehra I, Nadeem S, Abbas N, SaleemA Issakhov A (2020) Influence of Lorentz force and induced magnetic field effects on Casson Micropolar nanofluid flow over a permeable curved stretching/shrinking surface under the stagnation region. Surf Interfaces 2:100766. https://doi.org/10.1016/j.surfin.2020.100766
Nadeem S, Abbas N (2019) Effects of MHD on modified nanofluid model with variable viscosity in a porous medium. Nanofluid Flow in Porous Media. https://doi.org/10.5772/intechopen.84266
Aroke UO, Abdulkarim A, Ogubunka RO (2013) Fourier-transform infrared characterization of kaolin, granite, bentonite and barite. ATBU J Environ Technol 6:42–53
Dim PE, Mustapha LS, Termtanun M, Okafor JO (2021) Adsorption of chromium (VI) and iron (III) ions onto acid-modified kaolinite: isotherm, kinetics and thermodynamics studies. Arab J Chem 14:103064. https://doi.org/10.1016/j.arabjc.2021.103064
Esvandi Z, Foroutan R, Peighambardoust SJ, Ramavandi B, Akbari A (2020) Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surf Interfaces 21:100754. https://doi.org/10.1016/j.surfin.2020.100754
Das B, Mondal NK (2011) Calcareous soil as a new adsorbent to remove lead from aqueous solution: equilibrium, kinetic and thermodynamic study. Univ J Environ Res Technol 1(4):515–530
Abd El-Azim H, El-Sayed Seleman MM, Saad EM (2019) Applicability of water-spray electric arc furnace steel slag for removal of Cd and Mn ions from aqueous solutions and industrial wastewaters. J Environ Chem Eng 7:102915. https://doi.org/10.1016/j.jece.2019.102915
Jung K, Lee S, Lee Y (2018) Hydrothermal synthesis of hierarchically structured bir- nessite-type MnO2/biochar composites for the adsorptive removal of Cu(II) from aqueous media. Bioresour Technol 260:204–212. https://doi.org/10.1016/j.biortech.2018.03.125
Vojoudi H, Badiei A, Bahar S, Ziarani G, Faridbod F, Ganjali M (2017) Post-mod-ification of nanoporous silica type SBA-15 by bis (3-triethoxysilylpropyl) tetrasulfide as an efficient adsorbent for arsenic removal. Powder Technol 319:271–278. https://doi.org/10.1016/j.powtec.2017.06.028
Barka N, Qourzal S, Assabbane A, Nounah A, Ait-Ichou Y (2011) Removal of reactive yellow 84 from aqueous solutions by adsorption onto hydroxyapatite. J Saudi Chem Soc 15:263–267. https://doi.org/10.1016/j.jscs.2010.10.002
Baig JA, Kazi TG, Shah AQ, Kandhro GA, Afridi HI, Khan S, Kolachi NF (2010) Biosorption studies on powder of stem of Acacia nilotica: removal of arsenic from surface water. J Hazard Mater 178:941–948. https://doi.org/10.1016/j.jhazmat.2010.02.028
Gupta A, Balomajumder C (2017) Statistical optimization of process parameters for thesimultaneous adsorption of Cr (VI) and phenol onto Fe-treated tea waste biomass. Appl Water Sci 7:4361–4374. https://doi.org/10.1007/s13201-017-0582-9
Kumar P, Chauhan MS (2019) Adsorption of chromium (VI) from the synthetic aqueous solution using chemically modified dried water hyacinth roots. J Environ Chem Eng 7:103218. https://doi.org/10.1016/j.jece.2019.103218
Fu T, Niu Y, Zhou Y, Wang K, Mu Q, Qu R, Chen H, Yuan B, Yang H (2019) Adsorption of Mn(II) from aqueous solution by silica-gel supported polyamidoamine dendrimers: experimental and DFT study. J Taiwan Inst Chem Eng 97:189–199. https://doi.org/10.1016/j.jtice.2019.01.022
Chouchane T, Chouchane S, Boukari A, Mesalhi A (2015) Adsorption of binary mixture «Lead Nickel» by kaolin. J Mater Environ Sci 6:924–941
Niu Y, Qu R, Liu., Mu L, Bu B, Sun Y., Chen H, Meng Y, Meng L, Cheng L, (2014) Thiol-functionalized polysilsesquioxane as efficient adsorbent for adsorption of Hg(II) and Mn(II) from aqueous solution. Mater Res Bull 52:134–142. https://doi.org/10.1016/j.materresbull.2014.01.024
Feng X, Long R, Wang L, Liu C, Bai Z, Liu X (2022) A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep Purif Technol 284:120099. https://doi.org/10.1016/j.seppur.2021.120099
Mutar RF, Saleh MA (2021) Optimization of arsenic ions adsorption and removal from hospitals wastewater by nano-bentonite using central composite design. Mater Today Proc 60:1248–1256. https://doi.org/10.1016/j.matpr.2021.08.213
Yadav SK, Singh DK, Sinha S (2014) Chemical carbonization of papaya seed originated charcoals for sorption of Pb (II) from aqueous solution. J Environ Chem Eng 2:9–19. https://doi.org/10.1016/j.jece.2013.10.019
Yogeshwaran V, Priya AK (2021) Adsorption of lead ion concentration from the aqueous solution using tobacco leaves. Mater Today Proc 37:486–495. https://doi.org/10.1016/j.matpr.2020.05.467
Aksu Z, Tezer S (2005) Biosorption of reactive dyes on the green alga Chlorella vulgaris. Process Biochem 40:1347–1361. https://doi.org/10.1016/j.procbio.2004.06.007
El Aassar M, Fakhry H, Elzain AA, Farouk H, Hafez EE (2018) Rhizofiltration system consists of chitosan and natural Arundo donax L. for removal of basic red dye. Int J Biol Macromol 120:1508–1514. https://doi.org/10.1016/j.ijbiomac.2018.09.159
El Aassar MR, Masoud MS, Elkady MF, Elzain AA (2018) Synthesis, optimization, and characterization of poly (Styrene-co-Acrylonitrile) copolymer prepared via precipitation polymerization. Adv Polym Technol 37:2021–2029. https://doi.org/10.1002/adv.21860
Abdeen Z, Mohammad SG, Mahmoud MS (2015) Adsorption of Mn (II) ion on polyvinyl alcohol/chitosan dry blending from aqueous solution. Environ Nanotechnol Monit Manag 3:1–9. https://doi.org/10.1016/j.enmm.2014.10.001
Kan C-C, Aganon MC, Futalan CM, Dalida MLP (2013) Adsorption of Mn2+ from aqueous solution using Fe and Mn oxide-coated sand. J Environ Sci 25(7):1483–1491. https://doi.org/10.1016/S1001-0742(12)60188-0
Omri A, Benzina M (2012) Removal of manganese(II) ions from aqueous solutions by adsorption on activated carbon derived a new precursor: Ziziphus spina-christi seeds. Alex Eng J 51(4):343–350. https://doi.org/10.1016/j.aej.2012.06.003
Zhao J, Ye Z, Wang J, Cai G (2022). Adsorption of Mn2+ in simulated lake and reservoir water by modified biochar fixed bed. Environ Sci. 43(11). https://doi.org/10.13227/j.hjkx.202205315
Turp SM (2018) Mn2+ and Cu2+ adsorption with a natural adsorbent: expanded perlite. Appl Ecol Environ Res 16(4):5047–5057. https://doi.org/10.15666/aeer/1604_50475057
Payel S, Abul Hashem M, Hasan MA (2021) Recycling biochar derived from tannery liming sludge for chromium adsorption in static and dynamic conditions. Environ Technol Innov. 24:102010. https://doi.org/10.1016/j.eti.2021.102010
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Amer Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Carai IG, Jitareanui G (2015) Application of low-cost adsorbents for pesticide removal. Bull UASVM Agric 72(1):37–44. https://doi.org/10.15835/buasvmcn-agr:11182
Roux J, Brysont AW, Youngt B (1991) A comparison of several kinetic models for the adsorption of gold cyanide onto activated carbon. J South Afr Inst Min Metall 91:95–103
Bashir M, Tyagi S, Annachhatre AP (2020) Adsorption of copper from aqueous solution onto agricultural adsorbents: kinetics and isotherm studies. Mater Today Proc 28:1833–1840. https://doi.org/10.1016/j.matpr.2020.05.287
Zhuang H, Zhong Y, Yang L (2020) Adsorption equilibrium and kinetics studies of divalent manganese from phosphoric acid solution by using cationic exchange resin. Chin J Chem Eng 28:2758–2770. https://doi.org/10.1016/j.cjche.2020.07.029
Saleh TA (2015) Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): from surface properties to sorption mechanism. Desalination Water Treat 57(23):10730–10744. https://doi.org/10.1080/19443994.2015.1036784
Dutta SK, Amin MK, Ahmed J, Elias M, Mahiuddin M (2022) Removal of toxic methyl orange by a cost-free and eco-friendly adsorbent: mechanism, phytotoxicity, thermodynamics, and kinetics. S Afr J Chem Eng 40:195–208. https://doi.org/10.1016/j.sajce.2022.03.006
Saxena M, Kushwaha JP, Kulshreshtha S, Kaur G, Singh N (2022) Doxycycline adsorptive interaction with mesoporous MCM-41: kinetic and isotherm modelling with thermodynamics. Chem Afr 19(1–4):1055–1068. https://doi.org/10.1007/s42250-022-00365-w
Droepenu EK, Wee BS, Chin SF, Kok KY, Asare EA (2020) Adsorption of zinc oxide nanoparticles onto esterified carbonize sago hampas: kinetic and equilibrium studies. Iran J Mater Sci 17:152–169. https://doi.org/10.22068/ijmse.17.4.152
Chouchane T, Boukari A (2020) Removal of zinc in an aqueous solution by kaolin kinetic and thermodynamic studies. E J 5(3):676–691. https://doi.org/10.33826/etj/v5i3.01
Chouchane T, Chibani S, Khireddine O, Boukari A (2023) Adsorption study of Pb(II) ions on the blast furnace slag (BFS) from aqueous solution. Iran J Mater Sci Eng 20(1):1–13. http://ijmse.iust.ac.ir/article-1-3011-en.html
Chouchane T, Khireddine O, Chibani S, Boukari A (2023) Removal of Cr(III), Pb(II) and Cr-Pb mixture by blast furnace slag (BFS) in solution. Anal Bioanal Chem Res 10(3):251–268. https://doi.org/10.22036/ABCR.2022.365182.1843
Acknowledgements
The authors of the current work are sincerely thankful to the chemistry laboratory staff URMM/CRTI Annaba Algeria).
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
TC conducted the research and analyzed the data, and he wrote the paper; AB followed the experiences, OK analyzed the data, SC followed the experiences, and SC analyzed and interpreted the results. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Fig. S1. Effect of agitation speed: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S2. Effect of pH: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S3. Effect of temperature: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S4. Effect of particle size: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S5. Freundlich presentation: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S6. Langmuir presentation: (a) adsorption on kaolin, (b) adsorption on BFS. Fig. S7. Temkin presentation: (a) adsorption on kaolin, (b) adsorption on BFS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chouchane, T., Boukari, A., Khireddine, O. et al. Equilibrium, kinetics, and thermodynamics of batch adsorption of Mn(II) ions on blast furnace slag (BFS) and kaolin (KGA). J. Eng. Appl. Sci. 70, 58 (2023). https://doi.org/10.1186/s44147-023-00218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44147-023-00218-4