Abstract
Mucormycosis is a life-threatening infection caused by fungi in the Mucorales species. It mainly affects diabetes patients and other immune-compromised hosts. The infection can involve multiple organ systems, with the lungs being the second most common site. We report a case of a 40-year-old female who had a mass that resembled a tumor, a very rare manifestation of the disease which we were able to diagnose, treat, and now report as it is essential to initiate treatment as early as possible due to its aggressive behavior and high mortality rate.
Similar content being viewed by others
Background
Mucormycosis is caused by the fungus of the Mucorales order [1, 2]. Patients with diabetes mellitus (DM), hematological malignancies, solid organ transplants, and corticosteroid therapy are increasingly reported to have the infection. In the Asian continent, diabetes mellitus is the most commonly associated condition [3]. Mucormycosis has a high mortality rate [4,5,6]. Pulmonary mucormycosis is the third most prevalent manifestation of mucormycosis and is characterized by an acute, progressive, and fatal clinical course, with a mortality rate greater than 50% [1, 7]. Usually, bronchoscopic manifestations include mucosal necrosis, hyperemic mucosa, mass-like lesions, and purulent exudates, among other findings [8]. Diagnosis relies upon identifying non-septate hyphae in tissue by histopathology with culture confirmation [9]. L-AmB (liposomal amphotericin B) remains the first-line drug in mucormycosis therapy [10]. This report presents a case of pulmonary mucormycosis that mimicked a pulmonary tumor in a patient with uncontrolled diabetes mellitus type 2 (DM2), which is rarely seen.
Case presentation
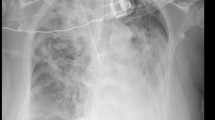
We report a case of a 40-year-old woman who presented with uncontrolled diabetes mellitus. She was diagnosed 3 years ago but did not adhere to her prescribed treatment regimen of 5 mg of glibenclamide twice daily and 500 mg of metformin after evening meals. She complained of dyspnea, productive cough with no hemoptysis, and generalized weakness for almost a month and underwent symptomatic treatment back in her small town. Her COVID test was negative. Her physical examination revealed no abnormalities, and her lung sounds were clear. Her initial blood glucose levels were between 300 and 400 mg/dL. She had a high ESR of 113mm/hand an elevated CRP of 25mg/dL for her age. Her initial spiral lung high-resolution computed tomography (HRCT) scan showed a collapse consolidation in the posterior segment of the right upper lobe (RUL) (Fig. 1). A diagnostic flexible fiberoptic bronchoscopy (FFB) confirmed a complete RUL obstruction due to a vegetative mass (Fig. 2). Histopathological study of the mass revealed features consistent with mucormycosis. Subsequently, The patient was admitted to the pulmonary section for definitive diagnosis and treatment. She was prescribed liposomal amphotericin B at a high dose (5 mg/kg/day) and intravenous (IV) insulin due to high blood sugar in her blood tests. Day after day, she showed improvement in her symptoms and maintained good glycemic control. After a week past her admission, we performed a paranasal sinus scan (PNS) CT, showing no signs of mucosal thickening or sinus opacification. On day 12 of her admission, to rule out a tumor malignancy superimposed by mucormycosis which we thought the case was, plus taking a larger and deeper sample biopsy, we performed a rigid bronchoscopy, which showed a significant decrease in the size of the endobronchial mass (Fig. 3). Tissue biopsy showed bronchial mucosa with foci of necrosis and numerous aggregate of broad non-septate fungal hyphae consistent with mucormycosis (Fig. 4), there we continued giving her liposomal amphotericin B (5 mg/kg/day). On day 15 of her admission, following her stable condition and constant improvement of symptoms, we did another spiral lung HRCT scan, showing an improvement from a mass-like tumor pattern to RUL infiltration with air bronchograms (Fig. 5). After 24 days past her admission, we discharged the patient while asking her to keep her sugar under control with 26 units of Lantus and keep taking her medications, which was 5 mg/kg/day of liposomal amphotericin B for 5 days, due to shortage of liposomal amphotericin B in Iran, and continuing with300 mg/12 h of Posaconazole for the 6th day and then using 300 mg daily for a month. After a month of follow-up, the patient appeared to be in complete remission with good glycemic control, and her blood tests showed a decreased ESR and CRP. We performed a follow-up spiral lung HRCT scan followed by FFB to see the patient’s response to treatment. The control spiral lung HTCT scan provided a huge decrease in size (Fig. 6), and the FFB showed an almost 95% decrease in her tumor-like mass, remaining less than 5% of mucosal thickening and vegetative inflammatory mass with fibrosis (Fig. 7). It has been two months of follow-up; the patient is asymptomatic, shows no clinical evidence of recurrent disease, and remains in a good glycemic control condition with low ESR, CRP, and normal-range WBC. No signs of amphotericin toxicity were observed.
Discussion
Mucormycosis is a severe, progressive disease with a high mortality rate that affects people of all ages. Susceptible individuals inhale fungal spores in the air or paranasal sinus, resulting in pulmonary mucormycosis [11]. The organisms are widely spread and typically infect individuals with diabetes mellitus or immune-compromised hosts [12]. Pulmonary infection is the third most common form of mucormycosis [9]. Pulmonary mucormycosis is a challenging case to diagnose; due to the lack of distinctive clinical manifestations, an aggressive clinical course challenges its management, and lack of data leading to treatment decisions, resulting in a high fatality rate of more than 50% [9, 12, 13]. In order to avoid misdiagnosis and delayed treatment, clinicians must make timely and accurate judgment calls regarding suspected cases. The present study presents an unusual manifestation of pulmonary mucormycosis, which has never been reported to the best of our knowledge. The clinical characteristics of pulmonary mucormycosis are non-specific. Some of the common ones include persistent high fever (> 38 °C), cough, hemoptysis, and chest pain [11]. In our case, the patient only had a productive cough with no hemoptysis. Radiological manifestations include infiltrates, exudation, consolidation, cavities, and nodules, while the disease typically has a predilection for the upper lobes [11, 14]. Usually, bronchoscopic manifestations include stenosis, erythematous mucosa, and obstruction of the airway [8]. Taking that into consideration, to the best of our knowledge, never before has anyone reported a tumor-like mass. Diagnosis relies upon identifying non-septate hyphae in tissue by histopathology [9]. In this case, the patient had a history of DM. Via clinical, radiological, and bronchoscopies along with histological examinations, we were able to diagnose a very rare case of mucormycosis, which is pulmonary mucormycosis presenting as a tumor-like mass. Treatment involves a combination of aggressive surgical debridement of involved tissues and antifungal therapy [5, 10]. The main reasons for not undergoing surgery in patients with pulmonary mucormycosis were the gravity of underlying diseases and concerns for operative risk [15]. Intravenous (IV) amphotericin B (lipid formulation) is the drug of choice for initial therapy [16]. The patient tolerated liposomal amphotericin B (5 mg/kg/day) well, without any signs of fever, chills, or gastrointestinal distress. The therapy was cost-effective and successful, leading to full recovery and discharge. Furthermore, managing any risk factors is one of the critical aspects of successfully treating pulmonary mucormycosis. The patient had a risk factor for diabetes, which required IV insulin administration during the amphotericin B therapy to keep the blood glucose level stable. Moreover, the kidney function, serum potassium level, and patient temperature were closely observed to enhance the therapeutic outcomes.
The interesting aspects of our case were the manifestation of pulmonary mucormycosis as a tumor-like mass, which can be misinterpreted with a tumor, whether it is from the lung or metastasized. Also, patients with solid tumors seldom suffer from secondary pulmonary mucormycosis [17], thus leading to a misdiagnosis or being overlooked by the attending doctor. Furthermore, this case reports a perfect regression of mucor mass after treating the patient with liposomal amphotericin B.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. We think our images suffice, but please do not hesitate to contact us for further data.
Abbreviations
- DM2:
-
Diabetes mellitus type 2
- RUL:
-
Right upper lobe
References
Jeong W, Keighley C, Wolfe R et al (2019) The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect 25(1):26–34. https://doi.org/10.1016/j.cmi.2018.07.011
Prakash H, Chakrabarti A (2021) Epidemiology of mucormycosis in India. Microorganisms. 9(3):523. Published 2021 Mar 4. https://doi.org/10.3390/microorganisms9030523
Prakash H, Chakrabarti A (2019) Global epidemiology of mucormycosis. J Fungi (Basel) 5(1):26. Published 2019 Mar 21. https://doi.org/10.3390/jof5010026
Smith JA, Kauffman CA (2012) Pulmonary fungal infections. Respirology. 17(6):913–926. https://doi.org/10.1111/j.1440-1843.2012.02150.x
Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G (2018) Challenges in the diagnosis and treatment of mucormycosis. Med Mycol 56(suppl_1):93–101. https://doi.org/10.1093/mmy/myx101
Soare AY, Watkins TN, Bruno VM (2020) Understanding Mucormycoses in the age of “omics”. Front Genet 11:699. Published 2020 Jun 30. https://doi.org/10.3389/fgene.2020.00699
He J, Sheng G, Yue H, Zhang F, Zhang HL (2021) Isolated pulmonary mucormycosis in an immunocompetent patient: a case report and systematic review of the literature. BMC Pulm Med 21(1):138. Published 2021 Apr 27. https://doi.org/10.1186/s12890-021-01504-8
He R, Hu C, Tang Y, Yang H, Cao L, Niu R (2018) Report of 12 cases with tracheobronchial mucormycosis and a review. Clin Respir J 12(4):1651–1660. https://doi.org/10.1111/crj.12724
Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP (2012) Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (Zygomycosis). Clin Infect Dis 54(Feb. (suppl_1)):S55–S60. https://doi.org/10.1093/cid/cir868
Brunet K, Rammaert B (2020) Mucormycosis treatment: recommendations, latest advances, and perspectives. J Mycol Med 30(3):101007. https://doi.org/10.1016/j.mycmed.2020.101007
Yang Y, Fang B, Xu X, Fang F, Pan M, Zhong X, Sun T (2013) Pulmonary mucormycosis: report of 5 cases and review of 46 cases reported in China. Chin J Tuberc Respir Dis 36:572–576 (In Chinese)
Skiada A, Lanternier F, Groll AH et al (2013) Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 98(4):492–504. https://doi.org/10.3324/haematol.2012.065110
Spellberg B, Kontonyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, Ibrahim AS, Brass EP (2012) Risk factors for mortality in patients with mucormycosis. Med Mycol 50:611–618
Yang C, Xie X (2015) Diabetes and nose-cerebral mucormycosis: A Case Study. Shiyong Yiyuan Linchuan Zachi 12:183–184 (In Chinese)
Choi H, Lee H, Jeon K, Suh GY, Shin S, Kim HK, Kim K, Jeong D, Kim H (2019) Factors affecting surgical resection and treatment outcomes in patients with pulmonary mucormycosis. J Thorac Dis 11(3):892–900. https://doi.org/10.21037/jtd.2019.01.75 PMID: 31019778; PMCID: PMC6462693
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, Mellinghoff SC, Mer M, Pana ZD, Seidel D, Sheppard DC, Wahba R, Akova M, Alanio A, Al-Hatmi AMS, Arikan-Akdagli S, Badali H, Ben-Ami R, Bonifaz A, Bretagne S, Castagnola E, Chayakulkeeree M, Colombo AL, Corzo-León DE, Drgona L, Groll AH, Guinea J, Heussel CP, Ibrahim AS, Kanj SS, Klimko N, Lackner M, Lamoth F, Lanternier F, Lass-Floerl C, Lee DG, Lehrnbecher T, Lmimouni BE, Mares M, Maschmeyer G, Meis JF, Meletiadis J, Morrissey CO, Nucci M, Oladele R, Pagano L, Pasqualotto A, Patel A, Racil Z, Richardson M, Roilides E, Ruhnke M, Seyedmousavi S, Sidharthan N, Singh N, Sinko J, Skiada A, Slavin M, Soman R, Spellberg B, Steinbach W, Tan BH, Ullmann AJ, Vehreschild JJ, Vehreschild MJGT, Walsh TJ, White PL, Wiederhold NP, Zaoutis T, Chakrabarti A, Mucormycosis ECMM MSG Global Guideline Writing Group (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19(12):e405–e421. https://doi.org/10.1016/S1473-3099(19)30312-3 Epub 2019 Nov 5. PMID: 31699664; PMCID: PMC8559573
Garcia-Covar Rubias L, Bartlett R, Barratt DM, Wassermann RJ (2001) Rhino-orbitocerebral mucormycosis attribu- table to Apophysomyces elegans in an immunocompetent individual: Case report and review of the literature. J Trauma 50:353–357
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. The authors confirm contribution to the paper as follows: study conception and design: Alireza Ziaei Moghaddam, Reza Basiri; data collection: Alireza Ziaei Moghaddam, Reza Basiri, Nema Mohamadian Roshan; draft manuscript preparation: Alireza Ziaei Moghaddam. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was composed in accordance with the criteria established by The Egyptian Journal of Bronchology to guarantee the quality of published case reports. Informed consent was obtained from the patient participating in our study.
Consent for publication
Informed consent was obtained from the patient participating in our study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ziaei Moghaddam, A., Basiri, R. & Mohamadian Roshan, N. Pulmonary mucormycosis presenting as a tumor-like mass in an uncontrolled diabetic patient: a rare case report. Egypt J Bronchol 17, 68 (2023). https://doi.org/10.1186/s43168-023-00248-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00248-9