Abstract
Background
Rheumatoid arthritis is considered one of the most common chronic inflammatory autoimmune diseases that lead to affection of several joints, as well as extra-articular organ involvement.
Rheumatoid arthritis women tend to menopause somewhat earlier. This was attributed to primary ovarian insufficiency because of autoimmune disorders. Anti-Müllerian hormone is a marker used for evaluating preantral follicle reserve. It provides a very sensitive way to reflect the ovarian reserve and has become a crucial factor in determining it.
The study aimed to show the influence of rheumatoid arthritis and its activity on ovarian reserve assessed using anti-Müllerian hormone serum levels.
Results
Our cross-sectional study involved 30 rheumatoid arthritis female patients with an age range between 25 and 35 years. The Disease Activity Score (DAS 28-ESR) was used to assess the degree of disease activity. Serum level of anti-Müllerian hormone was determined using quantitative enzyme-linked immunosorbent assay and the correlation with the disease activity as well as with the medications the patients were receiving was analyzed. A statistically significant relation was found amid the disease activity and the anti-Müllerian hormone level. Serum levels of anti-Müllerian hormone were found less in cases with high disease activity than in low to moderate cases. Different medications had no effect on anti-Müllerian hormone levels.
Conclusions
Rheumatoid arthritis high disease activity was linked to a diminished level of serum anti-Müllerian hormone.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is considered one of the most common chronic inflammatory autoimmune diseases that primarily affect several joints, leading to destructive polyarthritis. However, besides the articular involvement, RA has as well numerous extra-articular systemic manifestations. The etiology of RA is not fully known, however, several factors could play a role as genetic, environmental, and hormonal factors [1, 2]. RA affects up to 1% of the global population. Women are three times more vulnerable to develop RA than men. Moreover, women exhibit a greater chance of getting RA at a younger age. RA is a primary inflammatory disease that affects women in their childbearing period [3].
Extra-articular organ involvement in RA includes skin, eyes, renal, cardiopulmonary, nervous, and gastrointestinal systems in addition to ovaries [4]. Recent researches have revealed inexplicable infertility, with an extended mean time to conceive, higher predominance of dysovulation, nulliparous women, and diminished ovarian reserve in women with RA when compared to the general population. Almost one-third of RA female patients who are diagnosed during their early reproductive period undergo infertility issues [3, 5]. Many factors were suggested for these women’s subfertility such as disease inflammatory nature, disease duration, severity, and/or their treatments [5].
A significant reduction of ovarian reserve (OR) was noted in RA patients which corresponded to earlier menopause [6]. Studies showed that RA female patients tend to menopause earlier and that this was attributed to primary ovarian insufficiency because of autoimmune disorders [2, 4]. Valdeyron et al. reported a link between the development of RA and the incidence of early menopause before the age of 45 [5].
The preantral follicle reserve could be estimated by measuring the Anti-Müllerian hormone (AMH) in women’s serum. Research indicated that the amounts of this hormone are correlated with the sum of the evolving follicles in ovaries. Accordingly, serum AMH is looked up now as the most sensitive marker for OR, thus becoming a crucial factor in determining it [7].
Levels of AMH change throughout different life phases. Serum levels were found to be variable with a range of 0.2–11.7 ng/mL related to ovarian responsiveness [6]. At the average age of 50, a fixed drop to undetectable levels was noticed, consistent with menopause [8, 9]. The stability of AMH levels throughout the menstrual cycle provides the feasibility of its measurement at any time [10]. A threshold of < 1 ng/mL was regarded as evidence of marked diminution in ovarian reserve with affection of fertility [5].
Significant comorbidities such as polycystic ovarian syndrome (PCO) and some ovarian malignancies such as adult granulosa cell tumors could cause increased AMH [7, 11]. On the other hand, Anti-cancer therapies like cyclophosphamide, doxorubicin, and cisplatin were linked to premature ovarian insufficiency and infertility [12, 13].
Valdeyron et al. showed that inflammation might have a hazardous effect on the ovarian reserve and that there might be a link between the chronic inflammatory state present due to the cytokines and chemokines release and the decline of ovarian reserve, leading to early menopause [5].
Proinflammatory cytokines especially interleukin (IL)-6 and tumor necrotic factor alpha (TNF-α) which are secreted abundantly during the disease process, could lead to vessel damage, explaining the bond amid the inflammatory process and the ovarian reserve loss [2]. These patients showed an inverse relationship between AMH and IL-6 levels. Ovarian ischemic-reperfusion damage was attributed to the effect of TNF-α. High levels of TNF-α in ovaries lead to excessive leukocytic infiltration, injuring the ovarian tissue and causing follicular atresia and ovarian fibrosis [14, 15].
An animal experimental study showed that IL-1 was involved in ovarian reserve regulation through increasing AMH serum levels. Ovarian response to gonadotropins was noted to rise in IL-1-beta-lacking animals [16]. On the other hand, levels of IL-10 and vascular endothelial growth factor were found to stimulate angiogenesis and corpus luteum formation [17, 18].
Aim of the study
Our work aimed to show the influence of rheumatoid arthritis and its activity on ovarian reserve assessed using serum anti-Müllerian hormone levels.
Methods
Thirty RA female patients were involved in our cross-sectional study with ages ranging between 25 and 35 years. They were diagnosed with RA referring to the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for rheumatoid arthritis [19]. Those who had a history of PCOs, ovarian malignancies or surgeries, primary amenorrhea, gynecological radiotherapy, chemotherapy, any other autoimmune diseases, and also pregnant patients were excluded. This was confirmed through history and transabdominal pelvic ultrasound.
Subjects were enrolled in the outpatient clinic of the Rheumatology, Physical Medicine, and Rehabilitation Department over a period of 7 months. The nature of the research was explained to all participants. An informed consent was received from all of them. The faculty ethical committee approved the study.
All participants underwent full medical history taking with an emphasis on menstrual history, disease duration, and medications. A thorough clinical examination was done on all participants stressing both articular and extra-articular manifestations of RA. The degree of disease activity was evaluated according to the Disease Activity Score (DAS28-ESR). High activity was determined as > 5.1, moderate activity as 3.2–5.1, low activity as 2.6–3.2, and those with a score of < 2.6 were considered in remission [5].
The laboratory work-up included complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver and kidney function tests, and anti-Müllerian hormone serum level using quantitative enzyme-linked immunosorbent assay (ELISA).
For measuring serum levels of AMH, venous samples were collected and left for 10–20 min to coagulate at room temperature then centrifuged at the speed of 2000–3000 rpm for 20 min. The serum was then separated and stored at – 20 °C. The anti-Müllerian hormone-ELISA kit (Sun Red technology company) was used for measuring serum levels of AMH. The assay range was 0.08–20 ng/mL.
Statistical analysis
Statistical analysis of the collected data was done using the Statistical Package for Social Science (SPSS 20). Quantitative data were expressed as mean, standard deviation (± SD), median, and interquartile range (IQR). Non-numerical data were stated in the form of frequency and percentage. Student’s T-test was used for comparisons between means, while the Mann–Whitney U test was used for non-parametrical tests. The relationship between two qualitative variables was performed using the chi-square test. The correlation between two quantitative variables was determined using the Spearman correlation coefficient and method. A p value of < 0.05 was considered statistically significant.
Results
Descriptive data of the 30 recruited rheumatoid arthritis patients in our study is shown in Table 1. Twenty-three (76.7%) of our patients had regular cycles. All patients, except one, were multipara with no history of abortions or miscarriages. Only one patient was nulliparous.
The clinical findings of the recruited patients are summarized in Table 2. The summary of the medications our patients were receiving is listed in Table 3.
DAS28-ESR score was used to evaluate the degree of disease activity, and it ranged from 2.7 to 8.1 with a mean of 5.67 ± SD 1.60. Patients were further classified according to DAS degree, for which 18 patients (60.0%) had severe disease activity, 10 patients (33.3%) had moderate disease activity and only 2 (6.7%) of the patients showed mild disease activity. None of our patients was in remission (Table 4).
Laboratory investigations showed that 19 (63.3%) of our patients had microcytic hypochromic anemia. Regarding acute phase reactants, C reactive protein (CRP) ranged from 0.6 to 48 mg/dL with a median of 5 (2.2–9) and erythrocyte sedimentation rate (ESR) of the first hour ranged from 7 to 98 mm with a median 19.5 (10–41). All patients showed normal renal and liver function tests.
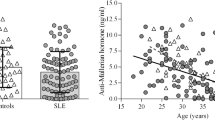
As an estimate of the ovarian reserve, serum level of anti-Müllerian hormone (AMH) was measured. Our subjects ranged between 0.75 and 2.9 ng/mL with a median of 1.6 (0.9–2) ng/mL. Patients were then categorized according to their AMH level into either AMH level ≥ 1 ng/mL or < 1 ng/mL. We found that 8 (26.6%) patients out of 30 showed an AMH level of > 1 ng/mL. While cases with AMH ≥ 1 ng/mL were 22 patients, 10 of them (45.5%) had high disease activity, 10 patients (45.5%) had moderate disease activity, and only 2 (9.1%) of them had low disease activity (Table 5 and Fig. 1). According to the literature, this level represents a consistently low level indicative of decreased ovarian reserve [5, 20, 21].
The negative correlation between serum AMH level and the DAS28-ESR score showed a high statistical significance (r = − 0.845, p = 0.000) (Fig. 2). Also, a highly statistically significant relation was detected among the activity degrees assessed by DAS28-ESR and the level of AMH (P value = 0.000). Less AMH levels were found in cases with high disease activity than low to moderate activity (Fig. 3).
A considerably statistically significant negative correlation was shown between AMH serum levels and age (r = − 0.622, p = 0.000). After excluding the age factor, there was also a significant negative correlation between serum levels of AMH and duration of illness (r = − 0.395, p = 0.031) (Table 6). No significant relation, however, was found between AMH serum levels and the different medications the patients were receiving.
Discussion
Rheumatoid arthritis (RA) is considered one of the most common autoimmune inflammatory diseases which is characterized, besides its destructive nature damaging several joints, by extra-articular manifestations counting the ovaries [4]. Numerous recent studies have proposed a high occurrence of subfertility in RA patients with diminished ovarian reserve when compared against normal people. Yet, causes are still indistinct or not settled yet [5, 22]. In our study, we aimed to show RA influence and its activity on ovarian reserve. The serum AMH level, known as the most sensitive indicator of ovarian reserve [7], was measured in 30 RA premenopausal Egyptian women.
AMH levels, in our study, were considered lower than the median range detected in the same age groups in the study conducted by Elattar et al. who measured AMH in 841 healthy Egyptian females providing an age-AMH nomogram and found that the median of AMH in the age group 25–29 years was 2.8 ng/mL and 1.88 ng/mL in the age group 30–34 years [23]. In accord with our study, Valdeyron et al. reported that the median of AMH level in 102 RA patients at the time of diagnosis was 1.7 ng/mL [5]. Henes et al. found that the median AMH value was 1.8 (0.0–4.3) ng/mL [6]. All these studies showed that RA subjects had significantly lower AMH when matched against the normal population within the same age group. Although Brouwer et al. in their study showed higher levels of AMH with a median of 2.5 (1.5–4.6) ng/mL [4], still, however, 45.0% of patients showed levels between the 10th and 50th percentiles and 34.9% were between the 50th and 90th percentiles of the healthy controls. Higher ranges, though, compared to our results might be due to the fact that younger patients with ages below 20 were included.
On the contrary, Brouwer et al. found that the median value of AMH levels was 1.71 (0.81–4.39) ng/mL in patients while 2.82 (1.64–4.38) ng/mL in the controls. Nevertheless, after adjusting the age factor, there were no statistically significant differences between the patients and controls. This controversy might be a result of the fact that they studied early RA cases [24]. Lopez-Corbeto et al. as well studied AMH serum levels in old established RA patients, and reported lower ranges of AMH with a median of 1.27 (0.42–2.24) ng/mL, showing no significant difference when compared to age-matched healthy controls. This could be clarified through the higher range of subjects age counted in in their study which was extended to 50 years old with a mean age of 37.4 ± 6.23 years in the two groups [25].
Eight (26.6%) of our patients showed AMH levels of (< 1 ng/mL). This comes in accordance with Henes et al. who showed that 30% of their RA cases had a reduced AMH level (< 1 ng/mL) which is suggestive of a constituently low level and thus a diminished ovarian reserve [6]. Brouwer et al. also stated in their study that eight out of their patients (11%) had AMH levels inferior to the tenth percentile of age-matched controls (< 1 ng/mL) [24]. In a recent study, Brouwer et al. stated that referring to age-specific percentiles, 50 RA patients had a serum AMH level beneath the 10th percentile at follow-up, compared to 20 patients at the first available assessment [2].
The highly statistically significant negative correlation between the DAS28-ESR scores and AMH levels came in accordance with Valdeyron et al. where they found that RA patients showed a high DAS28-ESR score on the first visit with a statistically significant negative correlation with AMH (r = − 0.12; P = 0.15). However, after receiving treatment and follow-up for 6 months, a statistically significant improvement of RA activity (r = − 0.28; P = 0.003) was achieved combined with a rise in AMH level (1.70 ng/mL vs. 2.20 ng/mL) for all patients assessed, whatever medications they received [5].
On the other hand, our results disagreed with Brouwer et al. whose both studies denied any link between serum level of AMH and disease activity [4, 2]. However, their studies were centered on a population with controlled disease activity and who had been participating in a pregnancy project. Recently, Lopez-Corbeto et al. as well did not find any association between disease activity and AMH levels (p > 0.5) [25]. This disagreement with our study may be due to the fact that their patients showed mild disease activity as their mean DAS28-ESR was 2.89 ± 1.54 compared to 5.67 SD ± 1.60 in our study.
As regards the relation between AMH classification whether < 1 ng/mL or ≥ 1 ng/mL and the degree of activity assessed by DAS28-ESR, a highly statistically significant relation was detected between them (p = 0.000). All 8 cases (100.0%) with AMH < 1 ng/mL were within the high DAS28-ESR group. As per our knowledge, no previous studies considered the relation between AMH < 1 ng/mL or AMH ≥ 1 ng/mL with the activity of DAS28-ESR. However, this result coincides with our hypothesis that high disease activity has a significant impact on AMH serum levels. As stated previously, this level was considered a markedly low level suggestive of decreased ovarian reserve [5, 20, 21].
The statistically significant negative correlation found between AMH serum level and age, in our study agreed with Henes et al. who found a statistically significant negative correlation between serum AMH and age in RA patients with a p-value of 0.009 [6]. Brouwer et al. also reported that patients with RA revealed a significant reduction in serum AMH levels with advanced age with a negative statistically significant correlation (p = 0.01) [2]. Moreover, Alexander et al. studied the effects of many rheumatic diseases as RA, juvenile idiopathic arthritis, and spondyloarthritis on AMH serum levels and found that a constant drop is noted beginning in the third decade and progressing till menopause [9]. Accordingly, Shrikhande et al. reported that combining both factors, AMH level and age, was a more consistent method to predict early menopause occurrence than age alone [11].
After excluding the age factor, AMH was found to have a statistically significant negative correlation with the duration of illness in our study. Brouwer et al. were in agreement that they found a faster drop of serum AMH levels over time in RA patients when compared to controls (p = 0.05) [2]. On the contrary, Lopez-Corbeto et al. denied any significant correlation between AMH serum levels and RA disease duration [25]. However, their study included subjects and controls up to 50 years old, in addition to the fact that average DAS was much lower than our study, where strict control might have contributed to mask the duration effect.
Regarding the influence of different medications on AMH level, no effects were detected either on patients receiving only MTX or on those getting MTX in addition to other treatments. Our study agreed with Brouwer et al. who held a follow-up study on RA patients receiving MTX over 6 months and found that AMH levels after age adjustment did not differ significantly among those receiving MTX and those who are not (p = 0.287) [24]. A similar result was observed by Eudy et al., Brouwer et al., and Lopez-Corbeto et al. [13, 4, 25]. Valdeyron et al. measured AMH at months 0, 6, 12, 24, and 36 post-diagnosis and did not note any considerable variance between AMH levels at months 12, 24, and 36 compared with month 0 (P = 0.05), whatever the medications received (MTX or not) [5].
The power of our study lies in the young age group of the RA patients who were recruited in the study. Extending our study by measuring other factors linked to ovarian reserve in larger samples could be a beneficial step. Moreover, evaluating cytokine levels in a trial to better understand how inflammatory processes induced by different cytokines in RA, could disturb the function of granulosa cells responsible for AMH production, could be beneficial and interesting.
Study limitations
Our study had some limitations represented by the small sample size, and the usage of only one parameter (AMH serum level measurement) for ovarian reserve assessment.
In conclusion, through this study, we were able to clarify the influence of disease activity in RA patients on AMH serum levels which in turn could be linked to a decrease in ovarian reserve and corresponding early menopause.
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR:
-
American College of Rheumatology
- AMH:
-
Anti-Müllerian hormone
- CBC:
-
Complete blood picture
- CRP:
-
C-reactive protein
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ELISA:
-
Enzyme-linked immunosorbent assay
- ESR:
-
Erythrocyte sedimentation rate
- EULAR:
-
European League Against Rheumatism
- IL:
-
Interleukin
- IQR:
-
Interquartile range
- JAK:
-
Janus kinase
- MTX:
-
Methotrexate
- OR:
-
Ovarian reserve
- PCO:
-
Polycystic ovarian syndrome
- RA:
-
Rheumatoid arthritis
- SPSS 20:
-
Statistical Package for Social Science
- TNF-α :
-
Tumor necrotic factor alpha
References
Karami J, Aslani S, Jamshidi A (2019) Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene 702:8–16. https://doi.org/10.1016/j.gene.2019.03.033. PMID: 30904715
Brouwer J, Dolhain R, Hazes J et al (2020) Decline of ovarian function in patients with rheumatoid arthritis: serum anti-Müllerian hormone levels in a longitudinal cohort. RMD Open 6(3):e001307. https://doi.org/10.1136/rmdopen-2020-001307
De Cock D, Brants L, Soenen I et al (2020) A systematic review on the effect of DMARDs on fertility in rheumatoid arthritis. Semin Arthritis Rheum 50(5):873–878
Brouwer J, Dolhain R, Hazes J et al (2019) Reduced ovarian function in female rheumatoid arthritis patients trying to conceive. ACR Open Rheumatol 1(5):327–335. https://doi.org/10.1002/acr2.11043
Valdeyron C, Soubrier M, Pereira B et al (2021) Impact of disease activity and treatments on ovarian reserve in patients with rheumatoid arthritis in the ESPOIR cohort. Rheumatology (Oxford) 60(4):1863–1870. https://doi.org/10.1093/rheumatology/keaa535. PMID: 33147613
Henes M, Froeschlin J, Taran F et al (2015) Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behçet’s disease and spondyloarthritis on anti-Müllerian hormone levels. Rheumatology 54(9):1709–1712. https://doi.org/10.1093/rheumatology/kev124
Li H, Robertson D, Burns C et al (2021) Challenges in measuring AMH in the clinical setting. Front Endocrinol 12:691432. https://doi.org/10.3389/fendo.691432
Dewailly D, Andersen C, Balen A et al (2014) The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update 20(3):370–385. https://doi.org/10.1093/humupd/dmt062
Alexander V, Ashley-Martin J, Riley J et al (2021) Association between arthritis treatments and ovarian reserve: a prospective study. Reprod BioMed Online 42(6):1203–1210. https://doi.org/10.1016/j.rbmo.2021.03.018
Kruszynska A, Slowinska-Srzednicka J (2017) Anti-Müllerian hormone (AMH) as a good predictor of time of menopause. Prz Menopauzalny 16(2):47–50. https://doi.org/10.5114/pm.2017.68591. Epub 2017 Jun 30. PMID: 28721129; PMCID: PMC5509971
Shrikhande L, Shrikhande B, Shrikhande A (2020) AMH and its clinical implications. J Obstet Gynecol India 70:337–341. https://doi.org/10.1007/s13224-020-01362-0
Spears N, Lopes F, Stefansdottir A et al (2019) Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 25(6):673–693. https://doi.org/10.1093/humupd/dmz027
Eudy M, McDaniel G, Hurd W et al (2019) Fertility and ovarian reserve among women with rheumatoid arthritis. J Rheumatol 46(5):455–459. https://doi.org/10.3899/jrheum.180176
Winger EE, Reed JL, Ashoush S et al (2009) Treatment with adalimumab (Humira?) and intravenous immunoglobulin improves pregnancy rates in women undergoing IVF. Am J Reprod Immunol 61:113–120. https://doi.org/10.1111/j.1600-0897.2008.00669
Kaplan S, Türk A (2020) Adalimumab increases follicle reserve and follicle development in rat ovary: the effect of adalimumab on ovarian reserve. Cureus 12(10):e11230. https://doi.org/10.7759/cureus.11230. PMID: 33269158; PMCID: PMC7704188
Olga MK, Kinga S, Natalie W-K et al (2022) Proinflammatory cytokines (IL-1, -6, -8, -15, -17, -18, -23, TNF-α) single nucleotide polymorphisms in rheumatoid arthritis—a literature review. Int J Mol Sci 23:2106. https://doi.org/10.3390/ijms2304210
Gómez RS, Busso SR, Garcia-Velasco C et al (2010) Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med 28(6):448–457
Çavus Y, Deger U (2020) Investigation of ADAMTS-1, BAX and IL-10 expressions in granulosa cells determinating fertilization success in IVF. Int J Morphol 38(2):427–434. https://doi.org/10.4067/S0717-95022020000200427
Aletaha D, Neogi T, Silman AJ et al (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Greene AD, Patounakis G, Segars JH (2014) Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet 31:935–946. https://doi.org/10.1007/s10815-014-0257-5
Cui L, Qin Y, Gao X et al (2016) Anti-Müllerian hormone: correlation with age and androgenic and metabolic factors in women from birth to postmenopause. Fertil Steril 105(02):481
Peña-Lizola SP, Sordia-Hernandez LH, Garcia-Luna SM et al (2021) Evaluation of ovarian reserve in women with rheumatoid arthritis. J Family Reprod Health 15(4):236–241. https://doi.org/10.18502/jfrh.v15i4.7889
El-Attar EA, Hosny TA, Ichihara K et al (2021) Nomogram of age-specific anti-Müllerian hormone levels in healthy Egyptian females. PLoS One 16(7):e0254858. https://doi.org/10.1371/journal.pone.0254858
Brouwer J, Laven J, Hazes J et al (2013) Levels of serum anti-Müllerian hormone, a marker for ovarian reserve, in women with rheumatoid arthritis. Arthritis Care Res 65:1534–1538. https://doi.org/10.1002/acr.22013. PMID: 23554429
Lopez-Corbeto M, Martínez-Mateu S, Pluma A et al (2021) The ovarian reserve as measured by the anti-Müllerian hormone is not diminished in patients with rheumatoid arthritis compared to the healthy population. Clin Exp Rheumatol 39(2):337–343. https://doi.org/10.55563/clinexprheumatol/73txen. Epub 2020 Sep 3 PMID: 32896242
Acknowledgements
To all patients included in this study for their co-operation, and also the department’s nurses and workers who assisted in the study processing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript All authors were involved in concept, design, data collection, analysis, and drafting the manuscript equally. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The faculty of Medicine Ain Shams University research ethics committee FMASU REC approved this research on 18/4/2022 (reference number: MS 328/2022). This research was conducted according to the standard of the Declaration of Helsinki and all participants signed written informed consent.is organized and operated according to the guidelines of the International Council on Harmonization (ICH).
Consent for publication
Not applicable.
The authors declare that this paper nor part of it has not been published or under publication elsewhere.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Israel, T., Saleh, H.A., Ahmed, S.F. et al. Anti-Müllerian hormone (ovarian reserve) in rheumatoid arthritis patients: correlation with disease activity. Egypt Rheumatol Rehabil 51, 28 (2024). https://doi.org/10.1186/s43166-024-00255-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-024-00255-8