Abstract
Background
Bone marrow edema syndrome (BMES), previously referred to as transient osteoporosis, is an uncommon and underdiagnosed self-limiting condition typically affecting the weight-bearing joints of the hip and lower limb. Its occurrence in upper limb or non-weight bearing joints is particularly rare.
Case presentation
We report the case of an otherwise healthy 42-year-old man who gradually developed severe and disabling left shoulder pain over the course of 6 months. Nine months after onset, he presented clinically with bilateral involvement and radiologically (magnetic resonance imaging, MRI) with diffuse BME associated with a subchondral insufficiency fracture, suggestive of proximal humeral avascular necrosis. Clinical evidence of subacromial shoulder impingement and MRI findings of bilateral subchondral bursitis and tenosynovitis with effusion of the biceps tendons likely resulted from primary BME, as musculoskeletal sonography confirmed the absence of rotator cuff tears. Repeated MRI findings at 2, 12, and 18 months documented near complete resolution of both edema and fracture, consistent with BMES of the proximal humerus. During this time, the patient reported a gradual improvement in both pain symptoms and range of motion. The clinical picture of insidious shoulder pain, exacerbated by activity and improved by load relief, in the absence of predisposing factors for osteonecrosis or antecedent trauma in patients of middle age, should indicate the possibility of the diagnosis of BMES.
Conclusions
This unprecedented report documents a rare case of bilateral BMES of the humeral head mimicking avascular necrosis. The correct diagnosis of BMES within an atypical anatomical location avoids invasive measures in the affected bone. The misdiagnosis of secondary BME and idiopathic osteonecrosis can be avoided by recognizing the characteristics of BME and subchondral fractures of the humeral head in the absence of rotator cuff tears, as well as their evolution on serial MRI.
Similar content being viewed by others
Background
Bone marrow edema syndrome (BMES) is a rare, painful condition of unknown etiology, mostly affecting middle-aged men (40–60 years old) and women during late pregnancy [1, 2]. BMES is a self-limiting condition associated with magnetic resonance imaging (MRI) evidence of diffuse BME and subchondral fractures [3]. Spontaneous pain caused by BMES is most often reported in the weight-bearing joints of the lower extremities. The most common sites, in descending order, are hip, knee, ankle, and foot [4]. BMES can appear at a single bone or multifocally and it is thought to resolve spontaneously after 6–24 months, although it may recur in another anatomical area [2, 5].
Primary BMES (including the migratory BMES variant) and transient BMES are interchangeable terms for the same pathological entity, also referred to as transient osteoporosis (with the corresponding migratory osteoporosis variant) before the advent of MRI [4, 5]. Transient osteolysis, transitory demineralization, and algodystrophy are alternative terms that refer to the same pattern of temporary painful BME, without any evidence of focal osteonecrosis or a specific underlying pathology [6]. Because of the low prevalence of the disease, the existing knowledge is based on single case reports or small case series. We describe a rare case of sequential bilateral upper arm presentation in a middle-aged man who was diagnosed with BMES of the humeral head mimicking avascular necrosis. The most common causes of bilateral shoulder pain include osteoarthritis, tendonitis, rotator cuff tears, and impingement syndrome. Based on the results of imaging and sonographic findings, the differential diagnosis was subsequently narrowed down to avascular necrosis and BMES, with the natural course of the condition confirming the latter.
Case report
We report the challenging diagnostic case of an otherwise healthy 42-year-old right-handed male physician who presented with gradually worsening left shoulder pain. The patient did not report either vocational or avocational activities that could result in overuse injuries. Following a spontaneous and insidious onset, the patient’s symptoms became increasingly more severe and incapacitating over the course of 6 months. The pain was invariably exacerbated by activity and weight-bearing, whereas it was temporarily alleviated by immobilization and load relief. Two courses of high-dose non-steroidal anti-inflammatory medication (Ibuprofen 1800 mg daily) proved ineffective and intractable pain-related sleep problems prompted referral to the orthopedic clinic.
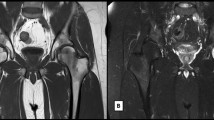
Following examination, based on the clinical suspicion of rotator cuff pathology, a conservative approach was recommended. An initial attempt at physiotherapy proved unfeasible, as a 2-month regimen of isometric exercises resulted in increased pain and functional impairment. The patient developed similar pain symptoms over his right shoulder about 9 months after contralateral onset. The results of full blood tests, including vitamin D level (65 nmol/L) and inflammatory parameters, were unremarkable. Inflammatory and other secondary causes of BME were ruled out. The patient was referred to bilateral shoulder MRI scan (Fig. 1).
The MRI study showed prominent BME involving the humeral head bilaterally, associated with a subchondral fracture at the central third of the humeral head (left shoulder) and a subchondral cyst formation within the posterior aspect of the humeral head (right shoulder). The imaging appearances were consistent with proximal humeral avascular necrosis. In consideration of the absence of known underlying causes for avascular necrosis, including history of trauma, alcohol and steroid use, the possibility of an idiopathic form of bilateral humeral head osteonecrosis was raised. At this stage, the overall shoulder function as assessed with the constant score [7]. Among the subjective variables of the constant score, pain was measured using a visual analog scale (VAS), whereas activities of daily living encompassed sleep, work, and recreation/sport [8]. With regard to the objective variables of the constant score, range of motion was established by goniometric examination of pain-free arm lifting across forward flexion, abduction, external and internal rotation, while strength was measured using the modified spring balance method [9]. For each Constant domain, the higher the score, the higher the quality of the function. The overall Constant score was 32 points, corresponding to a functional shoulder value of 33% of an age- and sex-matched normal control group [10]. Subscale scores were as follows: pain 5/15 points, activities of daily living 5/20, range of motion 16/40, strength 6/25. Shoulder examination demonstrated a positive Jobe’s test bilaterally; however, musculoskeletal sonography confirmed the absence of rotator cuff tears) [11].
Bilateral MRI of the shoulders was repeated 2 months later, following initial improvement of the left shoulder pain and persistent intertubercular bicipital groove point tenderness of the right shoulder (Fig. 1). There were residual pathological signs of a resolving subchondral insufficiency fracture (left shoulder) and persistent subchondral cystic change plus mild subacromial bursitis and tenosynovial fluid distension of the biceps long head tendon (right shoulder). Follow-up imaging data were obtained at 12 and 18 months. Repeated MRI findings documented that the previously demonstrated extensive BME in the humeral head had nearly completely resolved. The improvement was particularly noticeable at the level of the left humeral head, where the pathology had initially developed. Over time, there was a gradual resolution of associated findings (subchondral fracture and cyst), although the improvement of subacromial bursitis and tenosynovial fluid distension of the biceps long head tendon was considerably slower.
A further course of physiotherapy was recommended, including a combination of stretching and strengthening exercises, which allowed to regain a satisfactory range of motion. Gradual improvement in pain symptoms was accompanied by the development of catching and grinding sounds on active mobilization, as a result of functional changes related to the marked improvement of the bone marrow lesions with incomplete resolution of subacromial bursitis and tenosynovial fluid distension of the biceps long head tendon. Constant scores and subscores at follow-up (2, 12, and 18 months) are presented in Table 1. We obtained written consent for publication of data concerning the present case from the patient.
Discussion
The clinical picture of BMES was first described in 1959 in pregnant women, with the older name of transient osteoporosis of the hip [12], whereas its MRI correlate (BME) was first documented in 1988 [13]. BMES has since been described in other joints, affecting three times as many middle-aged men than women [6]. Patients with BMES report the spontaneous and insidious onset of severe, incapacitating pain that is exacerbated by activity and weight-bearing, and it is alleviated by immobilization and load relief [3, 14]. Temporal trajectories can vary, but pain symptoms usually increase over the course of several weeks, in the absence of any antecedent history of trauma. The plateau phase lasts for a couple of months, and then pain gradually declines over the following 3–9 months [15]. In some cases, complete resolution of symptoms can take up to 36 months [1, 6].
Painful BME can occur either spontaneously (primary BME or BMES) or in the context of underlying conditions encompassing almost all medical specialties (secondary BME). Consequently, before a diagnosis of BMES can be made, secondary causes of BME, including trauma, infection, tumor, metabolic imbalance, ischemia, joint inflammation, and degeneration, must be ruled out [4, 15, 16]. Specifically, the differential diagnosis of non-traumatic BME of the long bones encompasses osteoarthritis, rheumatoid arthritis, osteomyelitis, hydroxyapatite deposition disease, gout, bone cancer, and iatrogenic lesions (e.g., corticosteroids and other immunosuppressants). Bone marrow lesions such as BME are not visible on plain X-ray or computed tomography images. The differential diagnosis requires more specific imaging technologies, among which MRI and ultrasound yield the most useful information. The Ludwig Maximilians University LMU Consensus etiological classification of BME confirms that at present, BMES is a diagnosis of exclusion and can be distinguished from secondary causes of BME, particularly osteonecrosis, mainly by its self-limiting nature [4, 17]. In our case report, secondary BME was ruled out based on the patient’s clinical history, findings on examinations, and results of both laboratory tests and imaging investigations. Moreover, musculoskeletal sonography confirmed the absence of rotator cuff tears. These findings, combined with the time course, size, and distribution of the humeral head lesions, suggest that in our case report BME is unlikely to be due to shoulder impingement, although BME is often underestimated in the presence of soft tissue lesions. BMES is a rare condition and, consistently with its definition, its etiology remains largely unknown. The migratory variant of BMES (bilateral BMES) is suggestive of a disorder that may involve one or more joints, with the hip being the most common site [18, 19]. To the best of our knowledge, this rare condition has not been previously documented as affecting both humeral heads.
Although the cause of BMES is unknown, multiple etiopathogenic mechanisms have been proposed. The presence of a joint effusion in nearly all cases of BMES suggests synovial involvement, but targeted investigation has revealed only non-specific synovial fluid findings [15]. It has also been suggested that BMES could be a form of reflex sympathetic dystrophy [17, 20]. Finally, it has been hypothesized that BMES can develop as a result of a transient ischemic insult, suggesting that BMES and osteonecrosis may exist on a spectrum ranging from transient and reversible bone injury to extensive and permanent bone death. However, in a large study of 155 patients diagnosed with BMES of the hip by imaging criteria, none of the patients progressed to osteonecrosis, casting doubt on the suggestion that BMES is part of a clinical spectrum that results in either spontaneous resolution or avascular bone death [16]. Interestingly, none of the proposed pathogenic theories explain the increased incidence of BMES in middle-aged men or pregnant women.
In BMES, active osteoporotic changes have been associated with the presence of local subchondral microfractures (insufficiency fractures) and cysts [16, 21]. Radiography in the early stage of BMES does not register significant changes, requiring MRI analyzed by an experienced radiologist. MRI remains the examination of choice for the early diagnosis of BMES, as bone marrow lesions are not visible on plain X-ray or computed tomography images. MRI typically shows an ill-defined area of hypointense signal on T1-weighted images and hyperintense signal without sharp margins on T2-weighted images within the bone head; T2-weighted imaging may also reveal a joint effusion [17]. Overall, the MRI correlates of BMES are thought to be the expression of localized high bone turnover associated with repairing phenomena [22]. Although the altered MRI signal pattern is probably related to a displacement of normal fatty bone marrow by a more water-rich material or increased tissue vascularity within the trabecular bone, the actual histopathological mechanism of BMES remains unknown [5]. The absence of MRI signs of bone erosion or subchondral collapse, together with the self-limiting course, are helpful elements in the differential diagnosis between BMES and osteonecrosis (avascular necrosis).
Similarly to BMES, osteonecrosis has an uncertain pathophysiology, with 10% of cases being idiopathic and 80% being caused by excessive use of alcohol and use of steroids [23]. Contrary to BMES, osteonecrosis has a progressive course which often requires invasive surgical interventions [24]. However, the differential diagnosis between BMES and idiopathic osteonecrosis can be challenging, since initial MRI findings are often non-specific and there are no biomarkers or other laboratory tests that are helpful in confirming the diagnosis of BMES [3]. The clinical presentations in the early stages of BMES and osteonecrosis show considerable overlaps, with bilateral involvement in a substantial proportion of patients. Moreover, humeral head osteonecrosis is easily masked by other more common diagnoses and concomitant conditions [25]. Specifically, cases of idiopathic humeral head osteonecrosis mimicking rotator cuff disorders have been reported, although the BME pattern that can be observed in some cases of rotator cuff pathology tends to be confined to the fibro-osseous junction around the tendon insertion area [24]. There is also the possibility that either BMES or osteonecrosis co-occur with rotator cuff tears: evidence from a mass screening study showed that the prevalence of rotator cuff tears in the general population was 22%, with higher prevalence figures in older people and asymptomatic tears being twice as common as symptomatic tears [26].
Evidence-based guidelines for the diagnosis and management of BMES are lacking [4]. Since BMES is a self-limiting condition, conservative treatment should aim at reduction of pain and disability, as well as dissolution of the BME. Measures to shorten the natural history of BMES include reduced weight-bearing, immobilization of the affected area, analgesics and anti-inflammatory medication [2]. Additional treatment approaches have been suggested, including extracorporeal shock wave therapy, bisphosphonates, and iloprost, but conclusions regarding their efficacy have been precluded by the small number of cases and the lack of placebo-controlled trials [4]. The rarity of BMES makes it unlikely that randomized controlled trials assessing the therapeutic efficacy of known pharmacological agents will be conducted. Based on the available evidence, active monitoring of the clinical improvement could be complemented by further serial MRI investigations in case of unexpected changes during the recovery pathway. Further analyses of underlying molecular mechanisms are likely to provide new pathophysiological insights that might lead to novel, disease-specific therapeutic targets.
Conclusions
The clinical picture of insidious shoulder pain, exacerbated by activity and improved by load relief, in the absence of predisposing factors for osteonecrosis or antecedent trauma in patients of middle age, should indicate the possibility of the diagnosis of BMES. The present report shows that in selected cases, BME patterns on shoulder MRI can represent forms of BMES within an atypical anatomical location. Our rare case report is the first documentation of BMES affecting both humeral heads. The correct diagnosis of BMES of the humeral head, which improves slowly, though spontaneously, avoids invasive measures in the affected bone. The misdiagnosis of secondary BME and idiopathic osteonecrosis can be avoided by recognizing the characteristics of BME and subchondral fractures of the humeral head in the absence of rotator cuff tears, as well as their evolution on serial MRI. Further studies are warranted to elucidate endogenous pathogenetic factors related to BMES.
Availability of data and materials
Not applicable.
References
Solomon L (1993) Bone-marrow oedema syndrome. J Bone Joint Surg Br 75:175–176
Patel S (2014) Primary bone marrow oedema syndromes. Rheumatology 53:785–792
Berman N, Brent H, Chang G, Honig S (2016) Transient osteoporosis: not just the hip to worry about. Bone Rep 5:308–311
Baumbach SF, Pfahler V, Bechtold-Dalla Pozza S, Feist-Pagenstert I, Fürmetz J, Baur-Melnyk A, Stumpf UC, Saller MM, Straube A, Schmidmaier R, Leipe J (2020) How we manage bone marrow edema: an interdisciplinary approach. J Clin Med 9:551
Manara M, Varenna M (2014) A clinical overview of bone marrow edema. Reumatismo 66:184–196
Polesello G, Sakai DS, Ono NK, Honda EK, Guimaraes RP, Júnior WR (2015) The importance of the diagnosis of subchondral fracture of the femoral head, how to differentiate it from avascular necrosis and how to treat it. Rev Bras Ortop 44:102–105
Constant CR, Gerber C, Emery RJ, Søjbjerg JO, Gohlke F, Boileau P (2008) A review of the Constant score: modifications and guidelines for its use. J Shoulder Elbow Surg 17:355–361
Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J (2011) Measures of adult shoulder function. Arthritis Care Res 63(Suppl 11):174–188
Bankes MJ, Crossman JE, Emery RJ (1998) A standard method of shoulder strength measurement for the Constant score with a spring balance. J Shoulder Elbow Surg 7:116–121
Katolik LI, Romeo AA, Cole BJ, Verma NN, Hayden JK, Bach BR (2005) Normalization of the constant score. J Shoulder Elbow Surg 14:279–285
Leroux JL, Thomas E, Bonnel F, Blotman F (1995) Diagnostic value of clinical tests for shoulder impingement syndrome. Rev Rhum Engl Ed 62:423–428
Curtiss PH Jr, Kincaid WE (1959) Transitory demineralization of the hip in pregnancy. A report of three cases. J Bone Joint Surg Am 41-A:1327–1333
Wilson AJ, Murphy WA, Hardy DC, Totty WG (1988) Transient osteoporosis: transient bone marrow edema? Radiology 167:757–760
McWalter P, Hassan A (2009) Transient osteoporosis of the hip. Ann Saudi Med 29:146–148
Diwanji SR, Cho YJ, Xin ZF, Yoon TR (2008) Conservative treatment for transient osteoporosis of the hip in middle-aged women. Singap Med J 49:e17-21
Klontzas ME, Vassalou EE, Zibis AH, Bintoudi AS, Karantanas AH (2015) MR imaging of transient osteoporosis of the hip: an update on 155 hip joints. Eur J Radiol 84:431–436
Hayes CW, Conway WF, Daniel WW (1993) MR imaging of bone marrow edema pattern: transient osteoporosis, transient bone marrow edema syndrome, or osteonecrosis. Radiographics 13:1001–1011
Hofmann S, Engel A, Neuhold A, Leder K, Kramer J, Plenk H Jr (1993) Bone-marrow oedema syndrome and transient osteoporosis of the hip. An MRI-controlled study of treatment by core decompression. J Bone Joint Surg Br 75:210–216
Yi SR, Lee YH, Kim HM (2015) Bilateral bone marrow edema syndrome of the femoral head with a unique onset: a case report. Hip Pelvis 27:273–277
Froberg PK, Braunstein EM, Buckwalter KA (1996) Osteonecrosis, transient osteoporosis, and transient bone marrow edema: current concepts. Radiol Clin North Am 34:273–291
Guardiano SA, Katz J, Schwartz AM, Brindle K, Curiel R (2004) Fracture complicating the bone marrow edema syndrome. J Clin Rheumatol 10:269–274
Eriksen EF (2015) Treatment of bone marrow lesions (bone marrow edema). Bonekey Rep 4:755
Mont MA, Payman RK, Laporte DM, Petri M, Jones LC, Hungerford DS (2000) Atraumatic osteonecrosis of the humeral head. J Rheumatol 27:1766–1773
Kuo FY, Chen KL, Yen CC (2020) Idiopathic humeral head osteonecrosis mimicking rotator cuff disorders: two challenging diagnostic case reports. Medicine 99:e18766
Jackson SM, Major NM (2004) Pathologic conditions mimicking osteonecrosis. Orthop Clin North Am 35:315–320
Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, Kijima H, Itoi E (2013) Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: from mass-screening in one village. J Orthop 10:8–12
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The authors equally contributed to the drafting and final version of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cavanna, S., Cavanna, A.E. Bilateral bone marrow edema syndrome of the humeral head mimicking avascular necrosis: a case report. Egypt Rheumatol Rehabil 50, 29 (2023). https://doi.org/10.1186/s43166-023-00197-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00197-7