Abstract
Background
Familial hypercholesterolemia (FH) poses a substantial risk of cardiovascular diseases. The recent approval of evinacumab signifies a breakthrough in FH management. This review synthesizes evidence from diverse clinical trials, examining evinacumab’s efficacy, safety, and broader impact on hypercholesterolemia.
Body
As highlighted by multiple trials, Evinacumab demonstrates robust efficacy in reducing LDL-C levels, particularly in refractory cases. Its sustained impact, evidenced by enduring reductions in LDL-C levels throughout extended treatment periods, positions it as a potential long-term solution. While the safety profile appears favorable, instances of deaths underline the importance of holistic clinical management and ongoing surveillance. The clinical implications are profound, suggesting evinacumab’s potential inclusion in guidelines for managing severe lipid disorders.
Conclusion
Future research directions emphasize inclusivity, diversity, and real-world applications to establish sustained efficacy and safety across diverse populations. Integrating evinacumab into clinical guidelines requires evidence-based recommendations, necessitating collaboration between researchers, clinicians, and guideline developers.
Similar content being viewed by others
Introduction
Familial hypercholesterolemia (FH), a prevalent genetic disorder with an autosomal dominant pattern of inheritance, is characterized by markedly elevated blood low-density lipoprotein cholesterol (LDL-C) levels surpassing the normative threshold [1, 2]. FH patients exhibit distinctive lipid profiles, including heightened cholesterol levels, normal or diminished high-density lipoprotein (HDL), and often normal triglyceride (TG) levels, alongside an increased amount of LDL-C [3]. The clinical presentation of FH varies between heterozygous (HeFH) and homozygous (HoFH) forms, each posing unique challenges in diagnosis and management [4, 5]. Despite advances in therapeutic options, FH patients remain at an elevated risk of cardiovascular diseases, particularly coronary artery disease (CAD) [3]. Recognizing the significant impact of modifiable risk factors such as nutrition, exercise, and lifestyle, the management of FH necessitates a dual focus on maintaining a healthy routine and promptly initiating aggressive hypolipidemic medication [6].
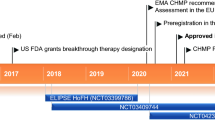
In this context, the recent approval of evinacumab by the European Union in February 2021 marks a milestone in hypercholesterolemia management [7]. Regeneron Pharmaceuticals developed evinacumab, an IgG4 monoclonal antibody targeting angiopoietin-like 3 protein (ANGPTL3) [8, 9]. Inhibiting ANGPTL3 unleashes the action of lipoprotein lipase (LPL) and endothelial lipase (EL), critical enzymes in lipid metabolism [10]. Utilizing genetically engineered recombinant Chinese hamster ovary cells, this humanized IgG4 anti-ANGPTL3 monoclonal antibody boasts two disulfide-linked human heavy chains and human kappa light chains, each with 453 and 214 amino acid residues, respectively [11]. Evinacumab stands out as a promising therapeutic agent, employing a unique mechanism of action targeting angiopoietin-like 3 (ANGPTL-3) protein [12]. Evinacumab operates as a human monoclonal antibody specifically designed to interfere with the inhibitory effect of ANGPTL-3 on lipoprotein lipase (LPL), thereby enhancing lipid clearance and reducing circulating low-density lipoprotein (LDL) cholesterol levels [13].
ANGPTL3, a vascular endothelial growth factors (VEGF) family component primarily expressed in the liver, regulates lipid metabolism by inhibiting endothelial lipase and lipoprotein lipase activities [12, 13]. Loss-of-function variants in the ANGPTL3 gene are associated with reduced LDL cholesterol levels, emphasizing its significance as a therapeutic target [14]. Evinacumab’s multifaceted action begins by reducing ANGPTL3 activity, unleashing lipase activities, and facilitating lipoprotein hydrolysis, decreasing triglyceride (TG) levels [15, 16]. Forming an immune complex with evinacumab inhibits ANGPTL3, enhancing the removal of very low-density lipoprotein (VLDL) remnants and ultimately reducing LDL cholesterol levels via a mechanism independent of LDL receptors [10, 17]. This mechanism of action contrasts with other emerging strategies in cardiovascular medicine. For instance, gene silencing techniques utilizing antisense nucleotides to target ANGPTL-3 mRNA represent an innovative approach to modulating lipid metabolism at the genetic level. Additionally, the inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) has garnered significant attention, with monoclonal antibodies such as evolocumab and alirocumab demonstrating efficacy in lowering LDL cholesterol levels by preventing PCSK9-mediated degradation of LDL receptors [14] (Fig. 1).
While initial studies have demonstrated its efficacy in reducing LDL-C levels, the literature still needs to analyze its long-term impact and safety profile [11, 12]. Moreover, existing treatments may have limitations, including adherence issues, side effects, and variable responses among diverse patient populations. This review examines the efficacy, safety, and influence of novel evinacumab on FH. Understanding the long-term efficacy, safety profile, and broader impact of evinacumab on hypercholesterolemia is crucial for informing clinical decision-making and refining treatment strategies.
Methodology
To ensure a comprehensive review, relevant literature was searched. Electronic databases, including PubMed, MEDLINE, Embase, and Cochrane Library, were searched (see Table 1). The search terms included “evinacumab,” “hypercholesterolemia,” “LDL cholesterol,” “safety,” and “efficacy.” Additionally, manual searches of key journals and references from relevant articles were performed to identify additional studies.
Studies were included if they met the following criteria:
-
Published in English.
-
Examined the efficacy and safety of evinacumab in familial hypercholesterolemia.
-
Involved human subjects.
-
Provided relevant data on LDL cholesterol levels, cardiovascular outcomes, and safety profiles.
Studies were excluded if they were:
-
Case reports, reviews, or conference proceedings.
-
Studies with insufficient data on the outcomes of interest.
Data extraction was performed independently by two reviewers to ensure accuracy. Extracted information included study design, participant characteristics, intervention details, outcomes (including changes in LDL cholesterol levels, cardiovascular events, and adverse events), and follow-up duration.
A narrative synthesis approach was employed to summarise the findings due to the anticipated heterogeneity in study designs and outcomes. Key themes related to evinacumab’s long-term efficacy, safety, and influence on hypercholesterolemia were identified and presented.
Current evidence on efficacy and safety
A. Overview of efficacy
The efficacy of evinacumab has been studied across various clinical trials (see Table 2). In a pivotal clinical trial by Raal et al. (2023), 64 patients completing a 24-week intravenous evinacumab treatment exhibited a remarkable − 49.6% reduction in total LDL-P compared to -5.1% with the placebo [18]. The study design included two distinct periods to evaluate the efficacy and safety profile of Evinacumab, namely a double-blind treatment period of 24 weeks followed by an open-label treatment period for another 24 weeks. The LDL-C data from 58 patients showed a substantial mean reduction of 46.3% from baseline to week 48 with open-label evinacumab. Notably, in the double-blind treatment period the placebo arm showed more mean percent reduction in LDL-P by 55.8% compared to a 42.7% reduction in the evinacumab arm, underscoring the efficacy of evinacumab in LDL-P reduction [18]. The trial further elucidated the impact of evinacumab on diverse lipid profiles. In the overall population, there were significant reductions in HDL-C (mean, 0.4%), non-HDL-C (mean, 48.9%), total cholesterol (mean, 47.0%), ApoB (mean, 40.8%), triglycerides (median, 51.9%), and Lp(a) (median, 16.3%) from baseline to 48 weeks. Interestingly, the mean percentage reductions for patients with or without apheresis were 43.8% and 47.6%, respectively, suggesting consistent efficacy across patient subgroups. Similarly, Reeskamp et al. (2021) evaluated Evinacumab’s effect on two patients with null/null LDLR variants, showcasing promising results. Intravenous administration led to a significant reduction in pre-apheresis LDL cholesterol levels (2.48 ± 0.31 to 0.80 ± 0.16 mmol/l in patient A; 2.20 ± 0.13 to 0.78 ± 0.13 mmol/l in patient B) [19]. Follow-up CCTA revealed substantial regression in plaque burden, with patient A experiencing a 76% reduction and patient B an impressive 85% reduction in total plaque volume after 31.5 months, including 5 months of Evinacumab treatment. Reeskamp et al.’s study (2021) focuses uniquely on patients with null/null LDLR variants and utilizes CCTA for plaque assessment. While the study design adds novelty, the small sample size (two patients) raises concerns about statistical power. The absence of a control group limits the ability to attribute observed changes solely to evinacumab.
Banerjee et al. (2019) conducted a study assessing LDLR activity. Evinacumab, despite reducing LDL binding and uptake values, did not alter LDLR activity. This finding suggests that evinacumab’s efficacy may be independent of direct LDLR functional improvement [20]. Gaudet et al.’s (2017) open-label study involving nine adults offers insights into real-world applications [21]. The multifaceted lipid profile assessment adds depth, but the small sample size and lack of a control group limit generalizability and causal inference. In the single-group, open-label study, evinacumab demonstrated significant efficacy in nine adults with HoFH. The treatment led to a 49% reduction in LDL cholesterol levels at week 4, with concurrent decreases in apolipoprotein B, non-HDL cholesterol, triglycerides, and HDL cholesterol.
Frederick et al. (2021) investigated the combination of alirocumab and evinacumab in 25 patients with FH, reporting substantial reductions in LDL-C (− 62.9%), apolipoprotein B (− 54.9%), and triglycerides (− 54.5%) [22]. A study by Rosenson et al. (2023) demonstrated enduring reductions in LDL-C levels throughout 72 weeks with intravenous evinacumab [23]. It is noteworthy that Rosenson et al. 2023 explored the longer-term effects of Evinacumab in refractory hypercholesterolemic cases. A remarkable 45.5% reduction at the 72-week mark emphasizes its sustained efficacy, positioning evinacumab as a long-term solution for refractory hypercholesterolemia. Subcutaneous or intravenous administration of evinacumab in a trial involving 272 patients showcased promising longer-term efficacy. Subcutaneous administration led to a 10% reduction in LDL cholesterol levels at week 16 compared to placebo, while intravenous administration exhibited more than 25% reductions. Secondary efficacy analyses emphasised the consistency of evinacumab in lowering lipid levels, including a dose-dependent decrease in atherogenic lipoproteins and HDL cholesterol levels. A phase 3 trial by Raal et al. explored evinacumab’s efficacy in 65 patients with homozygous familial hypercholesterolemia, demonstrating a significant 47.1% reduction in LDL cholesterol levels compared to a modest 1.9% increase in the placebo group at week 24. The substantial between-group difference of 49% underscores the robust efficacy of evinacumab in lowering LDL cholesterol, addressing a critical need in patients with severe hypercholesterolemia [24].
B. Safety parameters and adverse event management
In the clinical trial conducted by Raal et al. (2023), treatment-emergent adverse events (TEAEs) occurred in 73.4% of patients during the Open-Label Treatment Period (OLTP), with the most common TEAEs being nasopharyngitis (9.4%) and headache (9.4%). Reeskamp et al. (2021) reported no adverse reactions during their study, indicating a favorable safety profile [18]. Similarly, Banerjee et al. (2019) found that evinacumab was well-tolerated, with no treatment discontinuations due to adverse events [20]. Gaudet et al.’s (2017) study involving patients with HoFH reported that all participants experienced at least one adverse event, with back pain (n = 4), nausea (n = 4), and nasopharyngitis (n = 2) being the predominant adverse events. Importantly, none of these events led to treatment discontinuation [22].
The safety of subcutaneous evinacumab was investigated in 266 patients in a study by Rosenson et al. (2021) [24]. Different doses and frequencies of administration were explored, and adverse events associated with intravenous administration of evinacumab were compared to those of a placebo group. Adverse events in the subcutaneous administration included diarrhea, headache, influenza-like illness, injection-site erythema, nausea, nasopharyngitis, and urinary tract infection, with varying occurrences based on different doses. Notably, the study by Mariko Harada-Shiba et al. (2021) involving healthy Japanese and Caucasian subjects revealed comparable profiles across different treatment groups [25]. However, there were slight variations between Japanese and Caucasian subjects in other dose groups. TEAEs were reported in 41.7% of subjects in the evinacumab group for the intravenous treatment groups, with similar rates across different IV dose groups. Hypersensitivity adverse events were experienced by a small percentage of subjects, with minimal differences between the placebo and evinacumab groups [23]. In the subcutaneous treatment groups, a higher incidence of TEAEs was observed in the evinacumab group compared to the placebo group, particularly in the 300 mg QW (once a week) group, where there was a higher frequency of TEAEs, especially hypersensitivity events. Notably, treatment-related TEAEs and injection-site reactions were specific to the 300 mg QW group. In Rosenson et al. study [23] severe adverse events were recorded in seven patients in evinacumab arm, although claimed by the investigators to be unrelated to the medications, they were coronary or cerebral atherosclerotic events, indeed the actual hard cardiovascular endpoints to be evaluated [23]. It is crucial to note that these fatalities were not attributed to the trial drug but were linked to underlying heart disease, without giving a logical explanation as to why mortality was higher in evinacumab arm, compared to the placebo despite having the same refractory hypercholesterolemia, which is considered a study limitation. The management approach did not involve specific interventions related to Evinacumab but focused on addressing the broader health condition contributing to the adverse outcome.
Clinical implications and future directions
The combined findings from various clinical trials on evinacumab's efficacy present significant implications for clinical practice, future research, and potential updates to clinical guidelines. Evinacumab stands out as a valuable addition to therapeutic options for patients with FH. Its consistent efficacy in reducing LDL cholesterol levels, particularly in refractory cases, suggests its potential role in managing challenging lipid disorders. The robust impact on LDL-P and other lipid profiles positions evinacumab as a potential breakthrough for patients with limited treatment options. Despite the promising findings, there is a need for ongoing research, particularly in the form of long-term studies with larger and more diverse populations. The current evidence, while compelling, often stems from trials with limited sample sizes, specific patient populations, and varying methodologies. Future research should focus on establishing sustained efficacy and safety profiles across different demographic groups, ensuring a more comprehensive understanding of evinacumab’s potential.
Pending further confirmation through rigorous research, evinacumab could influence guidelines for managing refractory hypercholesterolemia. Its unique mechanism of action demonstrated efficacy, and relatively favorable safety profile position it as a candidate for inclusion in clinical guidelines for treating severe lipid disorders. However, this recommendation should be substantiated by further robust evidence. The safety profile of evinacumab, as indicated by various trials, appears favorable. Treatment-emergent adverse events are relatively low, with the most common events being manageable and not leading to treatment discontinuation. This suggests that evinacumab is generally well-tolerated among diverse patient populations. Future research should investigate safety considerations, especially in prolonged and real-world use. Long-term safety data and post-marketing surveillance are essential to uncover any potential rare adverse events that may not be apparent in smaller clinical trials. Understanding the safety parameters in varied populations and different administration methods will contribute to a more comprehensive safety profile.
The instances of deaths reported in association with evinacumab underline the importance of a clinical approach. As evinacumab research advances, several key areas merit attention for future investigation and exploration. Future studies should prioritize inclusivity and diversity in patient populations to ensure the generalizability of findings across different demographic groups. This will contribute to a more comprehensive understanding of how evinacumab may benefit a broad range of individuals with severe hypercholesterolemia. Investigating real-world applications and outcomes of evinacumab beyond controlled clinical trial settings is essential. This includes exploring its effectiveness in routine clinical practice, potential challenges, and variations in response across different healthcare settings.
Limitations and strength of review
While our narrative synthesis provides valuable insights into the long-term efficacy, safety, and adverse events associated with evinacumab, it is important to note certain limitations. Primarily, the efficacy of evinacumab has been predominantly evaluated in terms of its impact on LDL lowering rather than directly assessing its effect on hard cardiovascular outcomes such as atherosclerotic cardiovascular complications. These complications include critical events such as the development of acute coronary syndrome, strokes, or peripheral atherosclerotic occlusive complications. Consequently, the absence of a direct evaluation of these outcomes within the current review limits full comprehension of the medication’s overall cardiovascular efficacy and safety profile. In addition, the variability of study designs and methodologies across the included trials is a limitation. The heterogeneity in participant characteristics, treatment regimens, and outcome measures introduces challenges in directly comparing results and drawing universally applicable conclusions. Additionally, several studies had small sample sizes, potentially impacting the statistical power and generalizability of findings. Moreover, the available evidence predominantly stems from relatively short- to medium-term trials, and there needs to be more data on the long-term impact of evinacumab. Understanding sustained efficacy and safety profiles over extended periods is crucial for informing clinical decision-making, especially in chronic conditions like familial hypercholesterolemia. Although this review acknowledges the efficacy of Evinacumab in the reduction of LDL cholesterol in familial hypercholesterolemia, the higher incidence of deaths in evinacumab arm in placebo-controlled RCT comparative studies mandates continued surveillance and holistic assessment of this medication before approving it.
Conclusion
The approval of evinacumab in the management of FH represents a pivotal advancement in addressing the challenges posed by this prevalent genetic disorder. This review explored the pharmacological underpinnings of evinacumab, shedding light on its mechanism of action as an IgG4 monoclonal antibody targeting angiopoietin-like 3 protein (ANGPTL3). The inhibition of ANGPTL3, a key regulator of lipid metabolism, demonstrates promise in addressing the distinctive lipid profiles observed in FH patients. Various clinical trials have underscored the current evidence on evinacumab’s efficacy, showcasing its ability to significantly reduce LDL cholesterol levels, particularly in challenging cases of refractory hypercholesterolemia. These trials not only emphasise the short-term impact on lipid profiles but also hint at sustained efficacy over more extended periods, positioning evinacumab as a potential long-term solution for patients with limited treatment options. However, the efficacy findings, while promising, call for continued research efforts to establish the sustained impact of evinacumab and its safety profile. The varied responses observed in different patient populations and the instances of deaths reported underscore the importance of ongoing surveillance and clinical management. Furthermore, the current evidence presents a strong case for future research to address the limitations of existing studies, including small sample sizes and specific patient populations, to ensure robust generalizability and applicability to diverse demographic groups, and to emphasize its safety in this vulnerable population of familial hypercholesterolemia.
The clinical implications of evinacumab are significant, heralding a new era in the therapeutic landscape for FH. Its unique mechanism of action, and consistent efficacy position it as a possible option in management protocols for familial hypercholesterolemia after further long-term studies to ensure safety. However, cautious optimism is warranted, pending further substantiation through rigorous research. Future research directions should focus on inclusivity, diversity, and real-world applications of evinacumab. Long-term studies with larger and more diverse populations are crucial for establishing sustained efficacy and safety profiles. The integration of evinacumab into clinical guidelines should be informed by evidence-based recommendations, emphasizing the importance of ongoing collaboration between researchers, clinicians, and guideline developers.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- FH:
-
Familial hypercholesterolemia
- LDL-C:
-
Low-density lipoprotein cholesterol
- HeFH:
-
Heterozygous Familial Hypercholesterolemia
- HoFH:
-
Homozygous Familial Hypercholesterolemia
- HDL:
-
High-density lipoprotein
- TG:
-
Triglycerides
- CAD:
-
Coronary artery disease
- ANGPTL3:
-
Angiopoietin-like 3 protein
- LPL:
-
Lipoprotein lipase
- EL:
-
Endothelial lipase
- VLDL:
-
Very low-density lipoprotein
- LDL-P:
-
Low-density lipoprotein particle
- ApoB:
-
Apolipoprotein B
- Lp(a):
-
Lipoprotein(a)
- CCTA:
-
Coronary computed tomography angiography
- IgG4:
-
Immunoglobulin G4
- VEGF:
-
Vascular endothelial growth factor
- SC:
-
Subcutaneous
- IV:
-
Intravenous
- TEAEs:
-
Treatment-Emergent Adverse Events
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9)
References
Jialal I, Barton DP (2016) Diagnosis of familial hypercholesterolemia: Table 1. Am J Clin Pathol 145:437–439. https://doi.org/10.1093/ajcp/aqw001
Nohara A, Tada H, Ogura M, Okazaki S, Ono K, Shimano K et al (2021) Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb 28:RV17050
Raal FJ, Hovingh GK, Catapano AL (2018) Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis 277:483–492. https://doi.org/10.1016/j.atherosclerosis.2018.06.859
Maliachova O, Stabouli S (2019) Familial hypercholesterolemia in children and adolescents: diagnosis and treatment. Curr Pharm Des 24:3672–3677. https://doi.org/10.2174/1381612824666181010145807
Shah NP, Ahmed HM, Wilson Tang WH (2020) Familial hypercholesterolemia: detect, treat, and ask about family. Cleve Clin J Med 87:109–120. https://doi.org/10.3949/ccjm.87a.19021
Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS (2017) Familial hypercholesterolaemia. Nat Rev Dis Primers 3:17093. https://doi.org/10.1038/nrdp.2017.93
Cartier JL, Goldberg AC (2016) Familial hypercholesterolemia: advances in recognition and therapy. Prog Cardiovasc Dis 59:125–134. https://doi.org/10.1016/j.pcad.2016.07.006
Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A et al (2012) Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease. J Am Coll Cardiol 60:2631–2639. https://doi.org/10.1016/j.jacc.2012.09.017
Civeira F, Arca M, Cenarro A, Hegele RA (2022) A mechanism-based operational definition and classification of hypercholesterolemia. J Clin Lipidol 16:813–821. https://doi.org/10.1016/j.jacl.2022.09.006
Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P et al (2020) Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 383(8):711–720. https://doi.org/10.1056/NEJMoa2004215
Warden BA, Duell PB (2021) Evinacumab for treatment of familial hypercholesterolemia. Expert Rev Cardiovasc Ther 19:739–751. https://doi.org/10.1080/14779072.2021.1955349
Engler C, Ebenbichler C (2021) Evinacumab to treat hypercholesterolemia. Drugs of Today 57:607. https://doi.org/10.1358/dot.2021.57.10.3317240
Surma S, Romańczyk M, KJ Filipiak (2021) Evinacumab - The new kid on the block. Is it important for cardiovascular prevention? Int J Cardiol Cardiovasc Risk Prev 11:200107. https://doi.org/10.1016/j.ijcrp.2021.200107. PMID: 34988554; PMCID: PMC8710419
Sosnowska B, Adach W, Surma S, Rosenson RS, Banach M (2022) Evinacumab, an ANGPTL3 inhibitor, in the treatment of dyslipidemia. MDPI. https://doi.org/10.3390/jcm12010168
Surma S, Romańczyk M, Filipiak KJ (2021) Evinacumab — an ANGPTL3 inhibitor; a new drug in the treatment of lipid disorders. Review on the literature and clinical studies. Folia Cardiol 16:30–9. https://doi.org/10.5603/FC.2021.0005
Kersten S (2019) New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr Opin Lipidol 30:205–211. https://doi.org/10.1097/MOL.0000000000000600
Reeskamp LF, Millar JS, Wu L, Jansen H, Harskamp DV, Schierbeek H, Gipe DA, Rader DJ, Dallinge-Thie GM, Hovingh GK, Cuche M (2021) ANGPTL3 inhibition with evinacumab results in faster clearance of IDL and LDL apoB in patients with homozygous familial hypercholesterolemia-brief report. https://doi.org/10.1161/ATVBAHA.120.315204
Raal RJ, Rosenson RS, Reeskamp LF, Kastelein JJP, Rubba P, Duell PB et al (2023) The long-term efficacy and safety of evinacumab in patients with homozygous familial hypercholesterolemia. JACC: Advances 2(9):100648
Reeskamp LF, Nurmohamed NS, Bom MJ, Planken RN, Driessen RS, van Diemen PA et al (2021) Marked plaque regression in homozygous familial hypercholesterolemia. Atherosclerosis 327:13–17
Banerjee P, Chan K, Tarabocchia M, Benito-Vicente A, Alves A, Uribe K et al (2019) Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arterioscler Thromb Vasc Biol 39(11):2248-2260.1
Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK et al (2017) ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med 377(3):296–297
Raal FJ, Rosenson RS, Kolovou G, Harada-shiba M, Mitchenko O, Rubba P, Ali S, Banerjee P, Khilla N, McGinniss J, Pordy R, Blom DJ (2021). Abstract 12027: Low-Density Lipoprotein Cholesterol in Patients With Homozygous Familial Hypercholesterolemia Who Participated in Sequential Studies of Alirocumab and Evinacumab. Circulation 144(Suppl_1). Available from: https://doi.org/10.1161/circ.144.suppl_1.12027
Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, Khilla N, McGinniss J, Gaudet D, Pordy R (2023) Longer-term efficacy and safety of evinacumab in patients with refractory hypercholesterolemia. JAMA Cardiol 8(11):1070–1076. https://doi.org/10.1001/jamacardio.2023.2921. PMID:37703006;PMCID:PMC10500429
Rosenson RS, Burgess LJ, Ebenbichler CF, Baum SJ, Stroes ESG, Ali S, Khilla N, Hamlin R, Pordy R, Dong Y, Son V, Gaudet D (2020) Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med 383(24):2307–2319. https://doi.org/10.1056/NEJMoa2031049. Epub 2020 Nov 15 PMID: 33196153
Harada-Shiba M, Ali S, Gipe DA, Gasparino E, Son V, Zhang Y, Pordy R, Catapano AL (2020) A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis 314:33–40. https://doi.org/10.1016/j.atherosclerosis.2020.10.013. Epub 2020 Oct 10 PMID: 33130482
Acknowledgements
None.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
GO conceptualized the study. All authors were involved in the literature review. NA and EK extracted the data from the reviewed studies. All authors wrote the final and first drafts. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olatunji, G., Kokori, E., Moradeyo, A.A. et al. A mini-review of efficacy, safety, and influence of novel evinacumab on familial hypercholesterolemia. Egypt J Intern Med 36, 69 (2024). https://doi.org/10.1186/s43162-024-00335-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00335-3