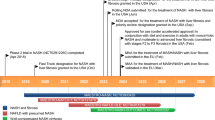
Abstract
The recombinant human monoclonal antibody evinacumab (evinacumab-dgnb, EVKEEZA™) is an angiopoietin-like protein three (ANGPTL3) inhibitor that has been developed by Regeneron Pharmaceuticals for the treatment of homozygous familial hypercholesterolaemia (HoFH), refractory hypercholesterolemia (both familial and non-familial) and severe hypertriglyceridaemia. Based on the results of the phase III ELIPSE HoFH trial, evinacumab was recently approved in the USA as an adjunct to other LDL-C lowering therapies for the treatment of adult and paediatric patients aged 12 years and older with HoFH, and has received a positive opinion in the EU. This article summarizes the milestones in the development of evinacumab leading to this first approval for HoFH.
Similar content being viewed by others
References
Regeneron Pharmaceuticals. FDA approves first-in-class EVKEEZATM (evinacumab-dgnb) for patients with ultra-rare inherited form of high cholesterol [media release]. 11 Feb 2021. https://investor.regeneron.com.
Regeneron Pharmaceuticals. Regeneron pipeline. 2021. https://www.regeneron.com/pipeline. Accessed 30 Mar 2021.
Gaudet D, Gipe DA, Pordy R, et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med. 2017;377(3):296–7.
Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med. 2020;383(8):711–20.
Regeneron Pharmaceuticals. EVKEEZA™ (evinacumab-dgnb) injection: US prescribing information. 2021. https://www.regeneron.com/sites/default/files/Evkeeza_PI.pdf. Accessed 15 Feb 2021.
European Medicines Agency. Evkeeza (evinacumab): summary of opinion (initial authorisation). 2021. https://www.ema.europa.eu/. Accessed 27 Apr 2021.
Regeneron Pharmaceuticals. Regeneron initiates major global collaboration with Sanofi-Aventis to develop and commercialize fully-human therapeutic antibodies [media release]. 29 Nov 2007. http://www.regeneron.com.
Sanofi Aventis. Sanofi-Aventis and Regeneron expand strategic antibody collaboration [media release]. 11 Nov 2009. http://www.sanofi-aventis.com.
Gusarova V, Alexa CA, Wang Y, et al. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. J Lipid Res. 2015;56(7):1308–17.
Harada-Shiba M, Ali S, Gipe DA, et al. A randomized study investigating the safety, tolerability, and pharmacokinetics of evinacumab, an ANGPTL3 inhibitor, in healthy Japanese and Caucasian subjects. Atherosclerosis. 2020;314:33–40.
Raal FJ, Rosenson RS, Reeskamp LF, et al. The longer-term efficacy and safety of evinacumab in patients with homozygous familial hypercholesterolemia [abstract no. 14407]. Circulation. 2020;142(Suppl 3):A14407.
Rosenson RS, Burgess LJ, Ebenbichler CF, et al. Evinacumab in patients with refractory hypercholesterolemia. N Engl J Med. 2020;383(24):2307–19.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Changes resulting from any comments received during the peer review process were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Evinacumab: First Approval. Drugs 81, 1101–1105 (2021). https://doi.org/10.1007/s40265-021-01516-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01516-y