Abstract
Background
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder with a high incidence of chronicity among adults. Rituximab is recommended as a treatment option for chronic ITP with the best long-term effect compared with other therapies. However, the optimal dose of rituximab remains unclear. We retrospectively compared the response rate, incidence of relapse, and other clinical outcomes in 34 chronic ITP patients who received rituximab in different doses. Patients were divided into two groups according to rituximab dose (the low-dose group, 100 mg/week for 4 weeks, and the standard-dose group, 375 mg/m2 weekly for 4 weeks).
Results
Females represented 72.2% and 56.3% of patients in the low dose and the standard dose groups, respectively. The overall response in all patients was 88.2%. By the end of the second month of treatment, 77.8% achieved partial remission and 11.1% achieved complete remission in the low-dose group versus 68.8% and 18.8% in the standard-dose group. Similar incidence of sustained complete response after 6 months of treatment, 83.3% after low dose rituximab and 81.2% after the standard dose (p = 1.000). The incidence of relapse was similar between both groups.
Conclusion
Our findings demonstrate that both regimens had no statistically significant differences in overall response, relapse rate, and time to reach response. The low dose of rituximab is comparable to the standard high dose in efficacy and safety for the treatment of chronic ITP patients and can be a good option in centers with limited resources.
Similar content being viewed by others
Introduction
ITP is an autoimmune disease characterized by low platelet counts and bleeding episodes of different severity caused by antibodies against platelet antigens. The International Working Group on ITP defines newly diagnosed patients within the first 3 months as acute ITP and those with disease duration between 3 and 12 months as persistent ITP, while patients with a duration of more than 12 months were considered chronic ITP [1]. Most children have acute ITP with a benign self-limiting course in 80% of cases. In contrast, adults rarely have spontaneous remissions and up to 75% develop chronicity which is associated with significant morbidities and challenging management. The chronic form affects individuals aged between 20 and 50 years and the incidence is higher among women than men, but this is reversed in older patients [2].
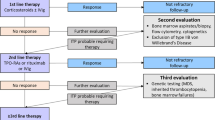
ITP is usually manageable with immunosuppressive therapy. Corticosteroids are considered the first-line treatment for newly diagnosed patients, but several known side effects of long-term therapy can occur even at lower doses. A subset of ITP patients still do not respond or relapse, some become refractory to one or more of the frontline therapies and require a second option for treatment [2, 3].
Splenectomy was considered as a second line in adults but pharmacological agents become an alternative in most cases. Multiple therapeutic approaches with proven clinical efficacy can be used in chronic ITP, including immunosuppressive drugs such as azathioprine, cyclosporine, and mycophenolate mofetil. Recently thrombopoietin receptor agonists (TPO-RA) and rituximab are the main second-line options [4].
Rituximab is a chimeric anti-CD20 monoclonal antibody that causes rapid but reversible depletion of CD20-positive B lymphocytes and modulation of T cells. It carries a more than 50% response rate. Different treatment schedules have been proposed, including the standard dose (375 mg/m2 weekly for 4 weeks) and a low dose regimen of 100 mg weekly for 4 weeks, with the advantage of low cost and potentially fewer adverse effects. However, the gold standard dose is still not known [5, 6]. The current study aims to compare the safety and efficacy of low-dose rituximab versus the standard dose in chronic ITP patients.
Patients and methods
A retrospective, single-center study was done on data obtained from a tertiary center’s registry. We reviewed the medical files of adult patients with chronic ITP who were diagnosed and followed up at the internal medicine department/hematology unit, Tanta University Hospitals, Egypt during the period from January 2021 to January 2023. Patients were diagnosed according to the criteria of the ITP International Working Group [7].
Patients aged ≥ 18 years who were intolerant, relapsed, or refractory to one or more treatment options and received rituximab as an alternative line of treatment were included. All patients had adequate performance scale, and negative serology for active/latent tuberculosis, Hepatitis B virus, and the human immune deficiency virus (HIV) infections. None of the patients tested positive for COVID-19 infection at the time of the pandemic.
Patients with thrombocytopenia secondary to other causes as systemic lupus erythematosus, hypersplenism, active H. pylori, or viral hepatitis infections were excluded. Pregnant women and patients with concomitant malignancies or recent infections were excluded. Those who received rituximab before due to other indications or received drugs that may affect platelet counts within ≤ 1 month before rituximab therapy were also excluded from the study, except patients maintained on low dose corticosteroids to keep the platelet count at least 20 × 109 /L to avoid severe bleeding.
Patients included in our study were divided into two groups based on rituximab dose:
-
Group 1 (low-dose rituximab): included chronic ITP patients who received rituximab at 100 mg as a weekly intravenous infusion for 4 weeks.
-
Group 2 (standard dose rituximab): included chronic ITP patients who received rituximab at 375 mg/m2 as a weekly intravenous infusion for 4 weeks.
Patients in both groups received additional prophylactic measures: intravenous hydrocortisone 100 mg, chlorpheniramine 10 mg or pheniramine hydrogen maleate 45.5 mg, and paracetamol 1000 mg as pre-medications were given 30 min before rituximab infusion as prophylaxis against infusion reactions. Pneumocystis Jirovecii infection prophylaxis with twice weekly trimethoprim-sulfamethoxazole, according to our local policy.
Detailed history has been reviewed, including data about age, sex, associated comorbidities, body mass index (BMI), duration of ITP, previous lines of treatment, and disease condition at the start of rituximab. Platelet counts, the severity of bleeding episodes using the ITP bleeding scale (IBLS) [8], and transfusion requirements at baseline and after treatment by rituximab were documented. Assessment of response to therapy was at 4 weeks and 6 months from the last infusion. Time to reach response, incidence of relapse, and therapy-related toxicities were documented. Results of routine laboratory tests such as complete blood picture, viral status, ANA, TSH, and H. pylori antigen in stool, liver, and renal functions were also reviewed.
Study definitions
The response was documented when the platelet count was ≥ 30 × 109/L. Complete response or complete remission (CR) when platelet count was ≥ 100 × 109/L measured two times more than a week apart in the absence of bleeding. Partial remission (PR) was defined in our study as a platelet count between 30 and 100 × 109/L taken on two occasions more than a week apart or double the baseline count in the absence of bleeding. No response if platelet count remains < 30 × 109/L or a less than twofold increase from baseline. Relapse was considered a drop of platelet count < 30 × 109/L after achieving response [7]. Refractory ITP in our study was defined when patients failed to achieve response after splenectomy or after more than one ITP treatment line in those who were contraindicated or refused splenectomy.
Statistical analysis
The collected data were analyzed using the SPSS software statistical computer package for Windows, version 25 (IBM Corp., Armonk, N.Y., USA). The Shapiro–Wilk for normality test was performed to assess the distribution of the numerical data. Normally distributed quantitative data were represented by mean, SD, and range. Abnormally distributed quantitative data were represented by mean, SD, range, and median. Qualitative data were presented by number and percentage. Independent t-test (t) was used for comparison between 2 independent groups regarding parametric quantitative variables. Mann Whitney U Test (U): for comparison between 2 independent groups regarding nonparametric quantitative variables. Friedman’s ANOVA: for comparison between more than 2 dependent groups regarding nonparametric quantitative variables. Marginal Homogeneity test (MH): for comparison between 2 dependent groups regarding qualitative variables. Pearson chi-square test (χ2): to detect whether there is a significant association between different categorical variables and when it was inappropriate, it was replaced by Fisher’s exact or Monte Carlo exact test. P value is used to indicate the level of significance when ≤ 0.05.
Results
The baseline characteristics and investigations of study participants were described in (Tables 1 and 2).
The age ranged from 19 to 50 in the low-dose group versus 23–46 years in the standard-dose group. The majority of patients were females in both groups. Few patients had comorbidities ranging from diabetes mellitus and hypertension which were complications from previous treatment with corticosteroids and only one patient had hypothyroidism but she was controlled on treatment. The duration of disease ranged from 12–24 to 12–30 months in the low-dose and standard-dose groups respectively. The majority in both groups had variable bleeding episodes before rituximab infusions, ranging from score 1 to 2 according to IBLS. All these differences between both groups were found statistically not significant.
The baseline platelet counts ranged from 13–25 to 8–27 × 109 /L in the low-dose and standard-dose rituximab groups, respectively with no statistically significant difference. All patients in the two groups had negative viral hepatitis except 3 cases were hepatitis C positive antibodies but their PCR was negative.
Treatment response and outcomes are described in (Tables 3 and 4). Among 34 chronic ITP patients in the present study; the initial response rate was assessed after 1 month from the last infusion, 88.9% of patients in the low dose group and 87.5% in the standard dose group were responders. CR and PR rates were similar between both groups. The sustained response after 6 months of treatment was complete in 83.3% (15 of 18 patients) and 81.2% (13 of 16 patients) in the low dose and the standard dose groups respectively. The median time to reach response was shorter in patients who received standard-dose rituximab than in the low-dose group (28 days versus 35 days). During follow-up after 6 months, four patients who received rituximab at a low dose and three patients in the standard dose group were relapsed. However, no significant difference could be detected between both groups.
Comparison between platelet counts and bleeding score at baseline and the end of 6-month post-treatment show overall significant improvement among our patients and also between patients in the same group but no significant difference was found between the two groups.
The adverse effects were generally mild and rituximab was a well-tolerated therapy in our patients, 72.2% of patients in the low-dose group and 75% in the standard-dose group underwent therapy without complications. Only 3 cases developed mild allergic reactions in both groups, other side effects including fever, fatigue, and myalgia were easily manageable by supportive therapy (Table 5).
Discussion
Primary ITP is a heterogeneous disease with an unpredictable evolution. Second-line treatments are usually needed to reach a hemostatic platelet count sufficient to reduce the risk of major bleeding and to improve the patient's quality of life [9, 10]. Currently, there are no sufficient studies to support the superiority of any treatment option as the variability in the treatment response is still not fully understood. The heterogeneity, different stages of the disease, and recently the metabolic alterations in bone marrow may explain the different therapeutic responses between patients [11, 12].
The American Society of Hematology (ASH) recommends the use of rituximab in patients with chronic ITP who want to avoid long-duration therapy or splenectomy [13]. In the present study; we compare the clinical outcomes of two groups of chronic ITP patients who received rituximab in two different doses.
All patients included in our study were relapsed or refractory to more than one previous line (47.1% and 52.9%) respectively. Treatments before rituximab varied among patients, corticosteroid alone or in combination with azathioprine ± vincristine were the most commonly used, followed by eltrombopag, mycophenolate mofetil, and splenectomy. The overall response was 88.2% among our patients with significantly higher platelet counts at one and 6 months after the last rituximab infusion compared with the baseline counts. Similar overall response and relapse rates were observed in both treatment groups. In this study, the low-dose rituximab regimen was associated with a lower incidence of hospital admission and platelet transfusion requirements. Most of the therapy-related side effects were mild and no death was reported in both groups.
Previous clinical studies demonstrated a considerably high response rate and relatively long sustained response in ITP patients following rituximab therapy [14]. The therapeutic mechanisms of rituximab in chronic ITP are mainly through depletion of CD20 + B cells, reduction of anti-platelet antibody production, and disturbed T cell activation. Most of the studies have given rituximab at 375 mg/m2 weekly for 4 weeks at the same doses that are recommended for B cell lymphomas. However, pharmacokinetic studies found that B cell burden in autoimmune diseases is much lower than B cell neoplasms; hence, it has been hypothesized that a low dose of rituximab is required for B cell depletion in autoimmune disorders, including ITP [15, 16].
Previous studies suggested that small-dose rituximab might be as effective as the standard dose in adults with ITP. Recent meta-analysis studies on chronic ITP patients by Li Y and Dong Y reported that the low-dose rituximab had similar overall response and relapse rates with fewer side effects and lower cost compared to the standard dose which was similar to our results [17, 18].
A similar sustained response rate at 6 months in both groups (88.9% and 87.5%) in the low-dose and standard-dose groups, respectively, this was in agreement with Kapoor R et al., who studied 21 chronic ITP patients who received rituximab at low dose. However, they reported that the median time to respond in their patients was 75 days, while in our patients it was 35 days in the low-dose group and 28 days in the standard-dose group [19].
In the current study, IBLS were significantly decreased after 1 and 6 months of treatment in the two groups. There was no statistically significant difference in bleeding scores between patients in both groups. This was similar to the findings published by a recent multicenter study that evaluated the efficacy of low-dose versus single high-dose rituximab. However; they reported a significantly higher incidence of hospitalization during treatment with low-dose rituximab which was unlike our findings [20].
Some studies investigated the effect of some patient factors on the response to rituximab as young age, female sex, shorter disease duration, and previous exposure to TPO-RA [21, 22] but we did not find this association among our patients in both groups. Similarly, studies by Kapoor R and Khellaf M supported our findings in this concern [19, 23].
The use of rituximab carries a possible risk of Hepatitis B reactivation and a small risk of progressive multifocal leukoencephalopathy, and COVID-19 vaccinations cannot be given for 6 to 12 months with its administration [5]. None of our patients developed such complications, and we considered delaying the vaccine for patients who received therapy during the pandemic.
Among our study participants, mild infusion reaction was documented in two patients in the low dose versus one patient in the standard dose group and all reported adverse effects were well tolerated. These findings are similar to data reported by Mishra K [24].
It should be noted that most of our patients received a combination of immunosuppressive drugs ± eltrombopag before receiving rituximab, although the ASH guidelines recommend its use as a primary option in chronic ITP. This can be explained by the high cost of rituximab in low-income countries like Egypt, which usually controls drug availability in many centers. So, we use these less expensive, readily available drugs, which are thought to have a good response. The advantage of immunosuppressive therapy in refractory severe ITP was also confirmed in previous studies, which reported a platelet count response in about 70% of patients treated with a combination of azathioprine, mycophenolate, or cyclosporine ± TPO-RA [25, 26].
Our study had some limitations: we did not provide data on the pharmacokinetics of rituximab as it was unavailable in a standardized lab in our country during the study period, also the small sample size and retrospective nature of the study. However, it was the first study conducted from our center on a homogenous group of patients with similar baseline characteristics and an accepted follow-up period evaluating the efficacy of low-dose rituximab in chronic ITP patients.
Conclusion
In developing countries, including Egypt, the accessibility of rituximab to a large group of patients remains a challenge due to financial restrictions. Based on our findings, both regimens were effective in achieving responses in chronic ITP patients who were relapsed/refractory to previous therapies. The low-dose rituximab had a similar response rate, improved the bleeding score, and offered a similar sustained response when compared to the standard dose, and it can be a good option with a lower cost. Additional prospective studies are indeed warranted to evaluate the long-term outcomes of a low-dose rituximab regimen with a longer follow-up after 12 months of therapy.
Availability of data and materials
Data are available upon request by contacting the corresponding author.
Abbreviations
- CR:
-
Complete remission
- ITP:
-
Immune thrombocytopenic purpura
- IBLS:
-
ITP bleeding scale
- PR:
-
Partial remission
- TPO-RA:
-
Thrombopoietin receptor agonists
References
Matzdorff A, Wörmann B (2018) Diagnosis and therapy of immune thrombocytopenia. Dtsch Med Wochenschr 143(15):1076–1081
Bussel J, Cooper N, Boccia R, Zaja F, Newland A (2021) Immune thrombocytopenia. Expert Rev Hematol 14(11):1013–1025
Vianelli N, Auteri G, Buccisano F, Carrai V, Baldacci E, Clissa C et al (2022) Refractory primary immune thrombocytopenia (ITP): current clinical challenges and therapeutic perspectives. Ann Hematol 101(5):963–978
Miltiadous O, Hou M, Bussel JB (2020) Identifying and treating refractory ITP: difficulty in diagnosis and role of combination treatment. Blood 135(7):472–490
Ghanima W, Khelif A, Waage A, Michel M, Tjønnfjord GE, Romdhan NB et al (2015) Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 385(9978):1653–1661
Zaja F, Volpetti S, Chiozzotto M, Puglisi S, Isola M, Buttignol S et al (2012) Long-term follow-up analysis after rituximab salvage therapy in adult patients with immune thrombocytopenia. Am J Hematol 87(9):886–889
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM et al (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):2386–2393
Page LK, Psaila B, Provan D, Michael Hamilton J, Jenkins JM, Elish AS et al (2007) The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol 138(2):245–248
Beyler O, Gunes A, Akat GK, Ceran F, Dagdas S, Ozet G (2020) The factors that affect the results of the response to rituximab treatment in ITP patients. Hematol Transfus Cell Ther 42:25
Mititelu A, Onisâi MC, Roșca A, Vlădăreanu AM (2024) Current understanding of immune thrombocytopenia: a review of pathogenesis and treatment options. Int J Mol Sci 25(4):2163
Jolink AT, Nelson VS, Schipperus MR, Amini SN, Vidarsson G, van der Schoot CE et al (2021) Potential diagnostic approaches for prediction of therapeutic responses in immune thrombocytopenia. J Clin Med 10(15):3403
Gilanchi S, Faranoush M, Daskareh M, Sadjjadi FS, Zali H, Ghassempour A et al (2023) Proteomic-based discovery of predictive biomarkers for drug therapy response and personalized medicine in chronic immune thrombocytopenia. BioMed Res Int 2023:9573863
Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N et al (2019) American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 3(23):3829–3866
Patel VL, Mahévas M, Lee SY, Stasi R, Cunningham-Rundles S, Godeau B et al (2012) Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 119(25):5989–5995
Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R (2007) Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica. 92(12):1695–8
Zaja F, Vianelli N, Volpetti S, Battista ML, Defina M, Palmieri S et al (2010) Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol 85(4):329–334
Li Y, Shi Y, He Z, Chen Q, Liu Z, Yu L, Wang C (2019) The efficacy and safety of low-dose rituximab in immune thrombocytopenia: a systematic review and meta-analysis. Platelets 30(6):690–697
Dong Y, Yue M, Hu M (2021) The efficacy and safety of different dosages of rituximab for adults with immune thrombocytopenia: a systematic review and meta-analysis. Biomed Res Int 2021:1–3
Kapoor R, Kumar R, Mahapatra M, Pati HP, Pramanik SK (2017) Low dose rituximab in chronic ITP: still an option in resource limited settings. Indian J Hematol Blood Transfus 33:568–573
Ni X, Li D, Yuan C, Yu Y, Wang H, Wang L et al (2022) Single-dose versus low-dose rituximab in corticosteroid-resistant or relapsed ITP: A multicenter, randomized, controlled study. Am J Hematol 97(4):440–447
Marangon M, Vianelli N, Palandri F, Mazzucconi MG, Santoro C, Barcellini W et al (2017) Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol 98(4):371–377
Bussel JB, Lee CS, Seery C, Imahiyerobo AA, Thompson MV, Catellier D et al (2014) Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica 99(7):1264–1271
Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard JF, Cheze S et al (2014) Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 124(22):3228–3236
Mishra K, Kumar S, Jandial A, Sahu KK, Sandal R, Ahuja A et al (2021) Real-world experience of rituximab in immune thrombocytopenia. Indian J Hematol Blood Transfus 37:404–413
Mahévas M, Gerfaud-Valentin M, Moulis G, Terriou L, Audia S, Guenin S et al (2016) Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood 128(12):1625–1630
Arnold DM, Nazi I, Santos A, Chan H, Heddle NM, Warkentin TE et al (2010) Combination immunosuppressant therapy for patients with chronic refractory immune thrombocytopenic purpura. Blood 115(1):29–31
Acknowledgements
The authors acknowledge the patients for their cooperative attitude in follow-up.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Mona M. Abdelaty and Dina G. El Saied wrote the manuscript and were responsible for the follow-up of patients during treatment. Amany M Dwidar was responsible for the exclusion of patients with thrombocytopenia due to viral hepatitis or splenomegaly. All authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted by the stipulations of the local ethical and scientific committee of Tanta University, Egypt. Ethics approval code: 36264PR443/11/23. Informed consent was provided by all patients authorizing the use of their data for research purposes.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Saied, D.G., Dwidar, A.M. & Abdelaty, M.M. Clinical efficacy of two different doses of rituximab as a treatment option in adult patients with chronic immune thrombocytopenia. Egypt J Intern Med 36, 60 (2024). https://doi.org/10.1186/s43162-024-00327-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00327-3