Abstract
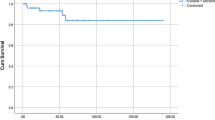
The etiology of ITP remains unknown but its pathogenesis consists of loss of tolerance to platelet antigens. There is a complex dysregulation of the immune system involving both the B cells and the T cells. Splenectomy is the standard second line option in steroid refractory chronic ITP patients. However, costs of surgery and reluctance for surgery in severely thrombocytopenic patients on part of surgeons are major obstacles in resource limited settings. Rituximab has been used in both the standard doses of 375 mg/m2 and low doses of 100 mg/m2 with similar results. We studied the utility of low dose Rituximab (@100 mg/m2 weekly × 4 doses) in resource limited settings. Overall response, complete response (CR) and partial response (PR) rates were 47.6% (10/21), 33.3% (7/21) and 14.3% (3/21) respectively. Median time to response in patients achieving CR was 75 days (range 45–185 days) while in patients achieving PR it was 105 days (range 45–165 days). However, there was no significant difference between males and females achieving CR or PR. We also observed that patients who had earlier responded to any form of treatment were more likely to respond to Rituximab treatment. The cumulative relapse free survival (RFS) at 13 months was 78%. By giving lower dose, six times less than conventional dosing dose, we have been able to demonstrate cost effectiveness in our study population. We were able to administer all the doses in day care without any major adverse events leading to further cost savings on in-patient care.

Similar content being viewed by others
References
Mueller-Eckhardt C (1977) Idiopathic thrombocytopenic purpura (ITP): clinical and immunologic considerations. Semin Thromb Hemost 3:125
Neunert C, Lim W et al (2011) The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 117(16):4190–4207
Moulis G, Sailler L (2014) Agne`s Sommet. Rituximab versus splenectomy in persistent or chronic adult primary immune thrombocytopenia: an adjusted comparison of mortality and morbidity. Am J Hematol 89:41–46
Arnold DM, Dentali F, Crowther MA et al (2007) Systematic review: efficacy and safety of Rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 146(1):25–33
Iacona I, Lazzarino M, Avanzini MA, Rupolo M, Arcaini L, Astori C et al (2000) Rituximab (IDEC-C2B8): validation of a sensitive enzyme-linked immunoassay applied to a clinical pharmacokinetic study. Ther Drug Monit 22:295–301
Provan D, Butler T, Amadori S, Newland AC et al (2007) Activity and safety profile of low-dose Rituximab for the treatment of autoimmune cytopenias in adults. Haematologica 92(12):1695–1698
Rodeghiero F, Stasi R, Gernsheimer T et al (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):2386–2393
R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Zhou Z, Yang R (2008) Rituximab treatment for chronic refractory idiopathic thrombocytopenic purpura. Crit Rev Oncol Hematol 65:21–31
Johnsen J (2012) Pathogenesis in immune thrombocytopenia: new insights. Hematology. Am Soc Hematol Educ Program 2012(1):306–312
Brændstrup P, Bjerrum OW, Nielsen OJ et al (2005) Rituximab chimeric anti-CD20 monoclonal antibody treatment for adult refractory idiopathic thrombocytopenic purpura. Am J Hematol 78:275–280
Patel VL, Mahévas M, Lee SY et al (2012) Outcomes 5 years after response to Rituximab therapy in children and adults with immune thrombocytopenia. Blood 119(25):5989–5995
Ghanima K, Waage A et al (2015) Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 385(9978):1653–1661
Tran H, Brighton T, Grigg A et al (2014) A multi-centre, single-arm, open-label study evaluating thesafety and efficacy of fixed dose Rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol 167:243–251
Zaja F, Battista ML, Pirrotta MT et al (2008) Lower dose Rituximab is active in adult patients with idiopathic thrombocytopenic purpura. Haematologica 93:930–933
Zaja F, Vianelli N, Volpetti S, Battista ML, Defina M et al (2010) Low-dose Rituximab in adult patients with primary immune thrombocytopenia. Eur J Hematol 85:329–334
Zaja F, Vianelli N, Battista M, Sperotto A, Patriarca F, Tomadini V et al (2006) Earlier administration of Rituximab allows higher rate of long lasting reponse in adult patients with autoimmune thrombocytopenia. Exp Hematol 34:571–572
Kelly K, Gleeson M, Murphy PT (2009) Slow responses to standard dose Rituximab in immune thrombocytopenic purpura. Haematologica 94(3):443–444
Zaja F, Baccarani M, Mazza P et al (2010) Dexamethasone plus Rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood 115(14):2755–2762
Li Y, Wang YY, Fei HR et al (2015) Efficacy of low dose Rituximab in combination with recombinant human thrombopoeitin in treating ITP. Eur Rev Med Pharm Sci 19:1583–1588
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors Dr. Rajan Kapoor, Dr. Rajiv Kumar, Prof. M. Mahapatra, Prof. Hara P. Pati and Dr. Suman Kumar Pramanik declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kapoor, R., Kumar, R., Mahapatra, M. et al. Low Dose Rituximab in Chronic ITP: Still an Option in Resource Limited Settings. Indian J Hematol Blood Transfus 33, 568–573 (2017). https://doi.org/10.1007/s12288-016-0764-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-016-0764-x