Abstract
We report a case of a 36-year-old female with untreated celiac disease, exhibiting both digestive discomfort and extra-digestive symptoms, including anemia. She subsequently developed a nephrotic syndrome. Biopsy of the accessory salivary gland confirmed amyloid deposits, as indicated by positive Congo red staining. Esophagogastroduodenal fibroscopy revealed characteristic signs of celiac disease and ruled out lymphoma. Further etiological screening identified celiac disease as the only possible cause of the amyloidosis. Complete remission was achieved after 3 months on a gluten-free diet, with later laboratory assessments indicating the absence of nephrotic syndrome and hematological abnormalities.
Similar content being viewed by others
Background
Celiac disease (CD) is a chronic inflammatory autoimmune disease of the intestine, Due to the ingestion of gluten in genetically predisposed subjects [1].
The gliadin found in gluten has a toxic effect acting directly on the intestinal epithelium causing recruitment of intraepithelial lymphocytes and an immunogenic effect on the T lymphocytes of the lamina propria.
These two effects are intertwined and induce an inflammatory reaction and tissue damage in the intestine.
Celiac disease generally takes the form of a malabsorption syndrome, which can manifest itself in different ways. The clinical presentation is frequently digestive (discomfort, diarrhea, bloating) but can also be extra-digestive: anemia symptoms, renal manifestations, osteopenia, and depression.
The gold standard for diagnosis involves serological tests and histological examination of duodenal biopsies.
Treatment is a total gluten avoidance. It results in the symptoms disappearance.
Case report
We report the case of a 36-year-old North African woman, suffering from celiac disease since the age of 10-year-old (diagnosis confirmed initially on an esophagogastroduodenoscopy and serological findings including positive IgA tissue transglutaminase and IgA anti-endomysial antibodies (Table 1). She is not adhering to a gluten-free diet, because of noncompliance, and experiences digestive discomfort, bloating, and diarrhea, and also extra-digestive signs, as microcytic and hypochromic anemia. She was admitted with pure nephrotic syndrome.
On clinical examination, the patient was conscious, in good general condition, apyretic, heart rate 81 bpm, blood pressure 100/60 mmHg, respiratory rate 19 cpm, and arterial oxygen saturation 99% on room air. Abdominal examination revealed moderate ascites, and the rest of the examination was normal.
Urine dipstick showed three-cross proteinuria, negative hematuria, and leukocyturia. Laboratory findings showed microcytic hypochromic anemia (hemoglobin: 6.8 g/dL) low ferritin, normal thyroid markers, normal renal function, hypoproteinemia at 45 g/L, and hypoalbuminemia at 16 g/L, with nephrotic-range proteinuria at 17 g/24 h (Tables 2, 3 and 4).
ANA, anti dsDNA, ANCA, APL, antiSM, antiSSA, antiSSB, and antiribosome antibodies were negative. Serum complement fractions C3 and C4 are normal (Table 1). Autoimmune disorders were then ruled out. Hepatitis B and C and HIV viral serology were negative (Table 5).
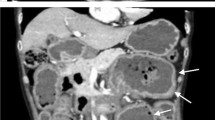
The patient underwent CT chest and abdomen imaging to search for infectious or malignant processes. Biopsies of the accessory salivary glands were performed and have shown amyloid deposits, Congo red-positive, and apple green birefringence under polarized light (Figs. 1, 2 and 3).
An esophagogastroduodenoscopy showed a reduction in the density of duodenal folds. Biopsies performed during it revealed total villous atrophy, with intraepithelial lymphocytes equaling 50%, and no evidence of lymphoma (Fig. 4).
The myelogram, bone marrow biopsy, electrophoresis, and serum protein immunofixation are not in favor of a lymphoma that could complicate celiac disease.
We performed an etiological screening of this amyloidosis, and no other cause was found. The nephrotic syndrome was finally attributed to renal amyloidosis due to her celiac disease. A gluten-free diet for celiac disease is started associated with renin-angiotensin-aldosteron system inhibitors. Total remission is achieved after 3-month diet. Laboratory findings show no nephrotic syndrome signs and no hematological disorders.
Discussion
Celiac disease (CD) is an autoimmune disease whose antigen is gluten. The frequency of CD in North Africa is close to that in Europe [2]. Its prevalence in the general population is about 0.5 to 1% [3]. Its prevalence remains underestimated, due to its generally silent nature and atypical expression [4]. Currently, the female-to-male ratio stands at 1.5:1 [5].
It can be associated with other autoimmune conditions, such as type 1 diabetes, autoimmune thyroiditis, autoimmune hepatitis, Sjögren’s syndrome, and IgA nephropathy [2].
CD is diagnosed clinically, serologically, and histologically and is confirmed by a favorable response to a gluten-free diet.
Extra-digestive manifestations of CD may be secondary to the resulting malabsorption syndrome or to autoimmune phenomena that have yet to be fully elucidated [6].
These include hematological, osteoarticular, neurological, cardiovascular, endocrine, immuno-allergic, and renal manifestations [7].
Renal involvement in CD is uncommon; the occurrence of celiac disease is elevated in individuals with chronic kidney diseases. While there is some debate surrounding this, there have been documented instances of an elevated risk of progressing to end-stage renal disease in individuals with celiac disease [7]. The most robust links are observed with IgA nephropathy [8] and diabetic nephropathy.
On the other hand, only a few sporadic cases of renal amyloidosis due to CD have been reported [1, 9]. Gluten gliadin penetrates the lamina propria massively. It triggers an inflammatory and immune response, which results in a release of inflammatory cytokines [10].
The latter are responsible of an increase in SAA protein levels, a major factor in amyloid deposits. Gluten-free diet is crucial in CD management; it can not only resolve symptoms but also prevent long-term complications (small intestinal adenocarcinoma, intestinal lymphoma) [11].
As for our patient, we obtained a full recovery (renal and extra-renal) after a 3-month well-respected gluten-free diet.
Conclusion
Celiac disease is essentially known for its digestive manifestations but can also cause extra-digestive signs, as renal ones. Hence, it is crucial to recognize the potential renal involvement in celiac disease.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CD:
-
Celiac disease
- HIV:
-
Human immunodeficiency virus
- ANA:
-
Antinuclear antibodies
- Anti dsDNA:
-
Anti-double-stranded DNA antibody
- ANCA:
-
Antineutrophil cytoplasmic antibodies
- APL:
-
Antiphospholipid
- AntiSM:
-
Anti-Smith antibody
- CT:
-
Computed tomography
- SAA:
-
Serum amyloid A
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- eGFR:
-
Estimated glomerular filtration rate
- IgG:
-
Immunoglobulin G
- IgA:
-
Immunoglobulin A
- IgM:
-
Immunoglobulin M
- TSH:
-
Thyroid-stimulating hormone
- CMV:
-
Cytomegalovirus
- EBV:
-
Epstein-Barr virus
References
Chhoda A, Jain D, Kumar Daga M, Batra V (2018) Celiac disease and secondary amyloidosis: a possible causal association? ACG Case Repo J 5:e24. https://doi.org/10.14309/crj.2018.24
Fasano A, Catassi C (2012) Celiac disease. N Engl J Med 367:2419–2426
Posner EB, Haseeb M (2023) Celiac disease. In: StatPearls. StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441900/
Caio G, Volta U, Sapone A et al (2019) Celiac disease: a comprehensive current review. BMC Med 17:142
Choung RS, Ditah IC, Nadeau AM et al (2015) Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol 110:455–461
Lauret E, Rodrigo L (2013) Celiac disease and autoimmune-associated conditions. Biomed Res Int 2013:127589. https://doi.org/10.1155/2013/127589
Therrien A, Kelly CP, Silvester JA (2020) Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol 54(1):8–21. https://doi.org/10.1097/MCG.0000000000001267
Cheung CK, Barratt J (2015) Gluten and IgA nephropathy: you are what you eat? Kidney Int 88(2):215–218. https://doi.org/10.1038/ki.2015.149
Kayhan B, Kuran OS, Turhan N, Akdoğan M, Sahin T (2003) Is celiac sprue an etiology of amyloidosis? Turkish J Gastroenterol 14(3):197–199
Malamut G, Cellier C (2010) Maladie Cœliaque. La Revue de Médecine Interne 31(6):428–433. ISSN 0248–8663
Jnawali P, Kumar V, Tanwar B (2016) Celiac disease: overview and considerations for development of gluten-free foods. Food Sci Human Wellness 5(4):169–176. https://doi.org/10.1016/j.fshw.2016.09.003
Acknowledgements
None.
Funding
There was no funding support for this study.
Author information
Authors and Affiliations
Contributions
ZK is involved in the clinical care of the patient, the drafting of the text, sourcing, editing of clinical images, writing the review, and editing. TB has revised the work. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
A consent was obtained from the patient for an anonymous publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaouiri, Z., Ftaimi, I., Benbella, M. et al. Renal amyloidosis: a hidden complication of celiac disease (a case report). Egypt J Intern Med 36, 26 (2024). https://doi.org/10.1186/s43162-024-00293-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00293-w