Abstract
Amyloidosis is a syndrome involving amyloid protein deposition in various organs, resulting in organ dysfunction. Symptoms of gastrointestinal amyloidosis are usually nonspecific, such as diarrhea and body weight loss. We, here, report a patient who presented to the hospital with simultaneous hematemesis, melena, and intestinal pseudo-obstruction, leading to a diagnosis of primary gastrointestinal amyloidosis based on computed tomography (CT) and endoscopic findings. CT showed diffuse wall thickening from the duodenum to the jejunum, jejunal dilation, and fluid accumulation throughout the gastrointestinal tract. Upper gastrointestinal endoscopy revealed duodenal mucosal edema, jejunal dilation, and small hemorrhages from jejunal mucosal erosion. The definite diagnosis was done based on biopsy results. This report describes the early diagnosis of gastrointestinal amyloidosis based on CT and endoscopy findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloidosis is a syndrome that involves amyloid protein deposition in various organs throughout the body, resulting in organ dysfunction [1]. Gastrointestinal amyloidosis includes amyloid A (AA) amyloidosis and amyloid light-chain (AL) amyloidosis [2]. The onset of gastrointestinal amyloidosis usually includes nonspecific symptoms, such as diarrhea and body weight loss; due to the variety of symptoms, diagnosis can be very difficult. Due to the limited number of reports, the association between gastrointestinal amyloidosis and complications, such as gastrointestinal bleeding and intestinal obstruction is not known. It is reported that abdominal computed tomography (CT) in patients with gastrointestinal amyloidosis sometimes show normal bowel findings, and if any abnormal findings are present, gastrointestinal wall thickening and bowel wall dilation will be seen [3].
Here, we report a patient who presented to the hospital with simultaneous hematemesis, melena, and intestinal pseudo-obstruction, leading to a diagnosis of primary gastrointestinal amyloidosis. This report appropriately describes the novel aspects of the study, including the early diagnosis made by CT and endoscopy findings.
Case report
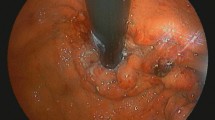
The patient was an 87-year-old female with a history of hypertension, type 2 diabetes mellitus (no medication, no neurological symptom, just follow-up), angina pectoris (she had medication such as β blocker and nitrate), and prior surgery for appendicitis. In early June 2016, she presented to our hospital emergency department with the primary complaints of hematemesis after eating, repeated vomiting, and melena. She was alert, and her body temperature was 36.7 °C. The blood pressure was 130/68 mmHg, and the regular heart rate was 76 beats/min. Her palpebral conjunctivae were indicative of anemia; there was tenderness in the epigastrium and peristaltic depression. A blood sample was collected (Table 1), and anemia and hypoproteinemia were detected. Abdominal CT showed diffuse intestinal wall thickening and fluid accumulation from the duodenal horizontal portion through the entire jejunum, and marked dilation of the proximal jejunum to a maximum diameter of 7.5 cm (Fig. 1). Although intestinal obstruction-like symptoms were confirmed, no obvious mechanism of obstruction could be identified; thus, a diagnosis of intestinal pseudo-obstruction was made. A clear trigger was not pointed out and a chronic progressive course was suspected. We considered malignant lymphoma, eosinophiric enteritis, SLE enteritis and Crohn disease as differential diseases. Upper gastrointestinal endoscopy was performed in emergency, duodenal mucosal edema, submucosal tumor-like protrusion, and duodenal dilation (Fig. 2) were observed. A biopsy was performed, and samples from the duodenum and jejunum stained with hematoxylin-eosin showed inflammatory cell infiltration, primarily of the plasma cells; amyloid deposition was visualized on staining with Congo red (Fig. 3). Immunostaining for amyloid A was negative, serum amyloid protein was low (5.0 µg/mL), and AL amyloidosis was diagnosed.
We performed lower gastrointestinal endoscopy 2 days later, but no inflammatory findings were observed and no abnormality was shown in biopsy. In addition, we examined small intestine with long scope and confirmed small ulcer and small bleeding in jejunum (Fig. 4). As a cause of melena, we speculated as duodenal erosion, small ulcer in jejunum, and small intestine that could not be observed. It was speculated that continuous mild bleeding caused the decrease the level of Hb. We considered the investing of small intestine.
After hospital admission, the treatment included refraining from intake of food orally, gastrointestinal pressure reduction by insertion of a stomach tube, and administration of proton pump inhibitors, resulting in the resolution of the patient’s symptoms. We administrated her medications that improve peristalsis and enteral feeding product. Following admission, she was discharged on day 5.
After the discharge, we managed the patient by administering intestinal motility improving medications and oral nutrients, and no recurrence of symptoms was observed.
Regarding the informed consent, we got the informed consent prior to inclusion in the study.
Discussion
There were two important clinical findings with respect to the patient reported here. She presented to the hospital with gastrointestinal amyloidosis, with the primary complaints of hematemesis, melena, and intestinal obstruction and the differential diagnosis of intestinal amyloidosis was predicted on the basis of characteristic CT findings.
First, the usual symptoms of gastrointestinal amyloidosis are body weight loss, diarrhea, constipation and fatigue [4]. In the database search, five cases of patients presenting with the primary complaint of hematemesis were found in the database of the Japan Medical Abstracts Society, and 22 cases were found on PubMed. With respect to intestinal pseudo-obstruction, nine cases were found in the database of the Japan Medical Abstracts Society, and 36 cases were found on PubMed [5, 6]. There are just three cases similar to the case presented here, reporting both, gastrointestinal hemorrhage and intestinal pseudo-obstruction [7,8,9] (Table 2). When amyloid protein deposition is primarily in the blood vessels, hemorrhage and hematoma formation occur, in addition to protein leakage. In contrast, intestinal peristaltic disorders are considered to develop in cases of amyloid protein deposition extending into the Auerbach’s plexus [10]. The coexistence of both gastrointestinal hemorrhage and pseudo-obstruction of the intestine should alert the clinician to a diagnosis of gastrointestinal amyloidosis.
Second, on the basis of characteristic CT findings, including diffuse wall thickening from the duodenum to the jejunum, jejunal dilation, and fluid accumulation throughout the gastrointestinal tract, we considered the potential diagnosis of intestinal amyloidosis before endoscopy was performed. Patients with AL amyloidosis, as in the present case, show thickening of the submucosal layer from the muscularis mucosa [11]. In contrast, in AA amyloidosis, the lamina propria is most prominently involved and in many cases, there is little noticeable wall thickening prior to this development [12].
The present patient had no significant underlying disease; thus, the diagnosis of primary AL amyloidosis was made. She had no symptoms related with amyloidosis such as neurologic abnormality, joint ache, anasarca, purple spot and enlarged lymph nodes. Electrophoresis, urinalysis, and cranial CT were performed, but findings suggestive of multiple myeloma were not identified, and rapid alleviation of symptoms was achieved with symptomatic treatment. Clinicians should consider the potential diagnosis of intestinal amyloidosis based on the presenting symptoms and characteristic CT findings.
References
Nienhuis HL, Bijzet J, Hazenberg BP. The prevalence and management of systemic amyloidosis in Western countries. Kidney Dis Basel Switz. 2016;2:10–9.
Cowan AJ, Skinner M, Seldin DC, et al. Amyloidosis of the gastrointestinal tract: a 13-year, single-center, referral experience. Haematologica. 2013;98:141–6.
Willekens I, Vandenbroucke F, Sennesael J, et al. Gastrointestinal amyloidosis presenting as enterocolitis on abdominal CT scan. J Radiol Case Rep. 2009;3:11–4.
Sattianayagam P, Gibbs S, Hawkins P, et al. Systemic AL (light-chain) amyloidosis and the gastrointestinal tract. Scand J Gastroenterol. 2009;44:1384–5.
Basavaraju KP, Mansour D, Barnes S, et al. A rare cause of dysphagia and gastroparesis. BMJ Case Rep. 2009;2009:bcr0720080358.
Tian L, Tang A, Zhang X, et al. Incomplete ileus and hemafecia as the presenting features of multi-organ involved primary systemic AL amyloidosis: a rare case report. BMC Gastroenterol. 2017;17:72.
Iwahashi N, Tame E, Nagasaka T, et al. Massive hemorrhage and pseudo-obstruction of the small intestine caused by primary AL amyloidosis associated with gastric cancer: report of a case. Surg Today. 2004;34:871–4.
Lau CF, Fok KO, Hui PK, et al. Intestinal obstruction and gastrointestinal bleeding due to systemic amyloidosis in a woman with occult plasma cell dyscrasia. Eur J Gastroenterol Hepatol. 1999;11:681–5.
Leong RY, Nio K, Plumley L, et al. Systemic amyloidosis causing intestinal hemorrhage and pseudo-obstruction. J Surg Case Rep. 2014;9:rju087.
Tada S, Iida M, Yao T, et al. Endoscopic features in amyloidosis of the small intestine: clinical and morphologic differences between chemical types of amyloid protein. Gastrointest Endosc. 1994;40:45–50.
Iida T, Yamano H, Nakase H. Systemic amyloidosis with gastrointestinal involvement: diagnosis from endoscopic and histological views. J Gastroenterol Hepatol. 2018;33:583–90.
Okuda Y, Yamada T, Ueda M, et al. First nationwide survey of 199 patients with amyloid A amyloidosis in Japan. Int Med. 2018;2018:1099–18.
Acknowledgements
This study was not supported by any fundings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yasutoshi Shiratori, Katsuyuki Fukuda, Takashi Ikeya, Koichi Takagi and Kenji Nakamura declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shiratori, Y., Fukuda, K., Ikeya, T. et al. Primary gastrointestinal amyloidosis with gastrointestinal hemorrhage and intestinal pseudo-obstruction: a report of a rare case. Clin J Gastroenterol 12, 258–262 (2019). https://doi.org/10.1007/s12328-018-00929-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-018-00929-9