Abstract
Aspergillosis is a common fungus that lives in soil and decaying vegetation. Inhalation of the spores causes infection mostly in immunocompromised patients. Invasive aspergillosis has an extremely high mortality, and a definitive diagnosis requires histopathological evidence of deep tissue invasion or positive culture; however, this evidence is often difficult to obtain due to the critical nature of the patients in these situations. The sensitivity of culture in this setting is also low. The galactomannan test is a recent antigen–antibody serologic test that depends on detecting an antigen which is a molecule found in the cell wall of aspergillus species. A positive result supports the diagnosis of invasive aspergillosis. We present a case of multiple intracerebral brain abscesses in an immunosuppressed patient due to an aspergillus species diagnosed by the galactomannan test with an excellent response to the treatment with the novel voriconazole alone, without any surgical intervention, and the purpose is to alert the physicians, neurologists, and infectious disease specialists to consider the intracranial aspergillosis among the differential diagnosis of the acute onset encephalitis especially in immunocompromised patients as early diagnosis and treatment may be life-saving.
Similar content being viewed by others
Background
Antifungal therapy alone for CNS aspergillosis has revealed disappointing results, with mortality of more than 90% in most series [1]. The most probable reason for such outcomes is poor penetration of antifungal drugs into the CNS; one exception to this rule is the novel antifungal drug voriconazole [2, 3]. We present a case known to have diabetes and hypertension, for more than 10 years, and on regular steroid therapy for autoimmune hemolytic anemia diagnosed 3 years prior to hospital admission. Because of fever and altered mentation, he was referred to the fever hospital, after failure of treatment with acyclovir for suspected acute viral encephalitis. The MRI shows multiple cerebral lesions at different areas of the brain with no appreciable enhancement in the post-contrast series, taking into consideration the history of being immunocompromised. The patient was on immune suppressive medications, lacked of response to acyclovir, and a positive galactomannan test in serum and CSF. The diagnosis of intracerebral aspergillosis was confirmed, voriconazole was started immediately without combining surgery or other antifungal medications. The outcome was excellent, and he was discharged after 1 month with minimal residual effects. It is important to mention here that in all the reported cases in the literature, the patients were treated with voriconazole combined with surgical intervention; in this case, only voriconazole was used and proved to be effective in achieving long-term favorable outcomes.
Case presentation
A 54-year-old Egyptian businessman was brought to the emergency room of Embaba-Fever Hospital (Giza, Egypt) in a semi-coma state after 4 days of headache, nausea, confusion, and increasing obtundation together with a high-grade fever. He was admitted to a private hospital where CSF analysis and multi-slice brain CT scan were done and then referred to the fever hospital as a case of CNS infection.
The patient is known to be diabetic and hypertensive, for more than 10 years, and 3 years earlier, he was diagnosed with autoimmune hemolytic anemia for which he was maintained on steroid therapy (prednisolone 20 mg daily). The relatives denied any history of recent or old trauma, animal contact or bite, or recent travel outside Egypt. There was no bowel or urinary disorder, and also, there was no remote or recent operative surgery.
The family reported a long history of frequent attacks of nasal stuffiness and headache relieved by nasal decongestants and simple analgesics. Four weeks before admission, he was transfused with packed RBCs for an attack of acute hemolytic crisis. There was associated fever, headache, dizziness, and diplopia, which gradually subsided by analgesics and antipyretics, and the family history was not contributory.
On examination, the patient’s Glasgow Coma Scale (GCS) was mild: 12/15 E4V4M4. He was noted to be underbuilt, pale, round pupils, regular and reactive, no jaundice, neck veins not congested, no lower limb edema, no visible skin rash, and no lymphadenopathy, and the neck was lax with no signs of meningeal irritation. The pulse was 100/m and regular, temperature 39 °C, and blood pressure 110/80 mmHg. There were no obvious swellings in the paraspinal region. Chest examination revealed scattered rhonchi over both lung fields. The heart was normal S1, S2. The abdomen was lax without any point of tenderness, no organomegaly, masses, or ascites, and renal angles were free.
The neurological examination showed no visible signs referring to cranial nerve affection. There was hypertonia with exaggeration of the deep tendon reflexes and positive Babinski’s sign.
The patient had a normal resting ECG
Initial investigations showed the complete blood count as follows (Table 1): normocytic, normochromic anemia, mild polymorphonuclear leucocytosis with absolute lymphopenia. All the biochemical, metabolic, and serological investigations were normal except the serum galactomannan test which came positive (Table 2). Serial CSF analysis was done to confirm laboratory improvement and to exclude persistent infection (Table 3).
The differential diagnosis includes the following:
-
Viral encephalitis
-
Bacterial meningoencephalitis
-
CNS tuberculosis
-
Cerebral toxoplasmosis
-
Intracerebral fungal infection by either mucormycosis or intracerebral aspergillosis
Meningoencephalitis (viral, bacterial, and tuberculous) has been considered; however, there were no signs of meningeal irritations (e.g., nuchal rigidity, Kernig’s sign, Brudzinski’s sign). Moreover, the lack of response to the antiviral acyclovir and the negative HSV PCR and negative gram stain and culture for bacteria excluded herpes simplex encephalitis and bacterial meningitis. Also, the course was too short for a diagnosis such as tuberculous meningitis, absence of cranial nerve affection, and the negative work-up for TB excluded tuberculosis meningitis, while the illness could explain some of the clinical manifestations of cerebral toxoplasmosis. The lack of history of HIV and the negative serology for toxoplasmosis are quite evidences to negate this disease.
Intracerebral invasive fungal infection by either mucormycosis or intracerebral aspergillosis was a reasonable diagnosis as both affect diabetics and immunocompromised patients. Invasive mucormycosis is clinically similar to invasive aspergillosis but is marked by angioinvasion and tissue infarction of the nose or oral cavity by the black necrotic eschar at the initial time of presentation. The clinical presentation was most compatible with the diagnosis of intracerebral aspergillosis. The concomitant history of nasal stuffiness and the radiologic findings that suggest fungal etiology in addition to the immunocompromised state of the patient make the diagnosis of intracerebral aspergillosis most likely.
The following features are consistent with the diagnosis of intracerebral aspergillosis:
-
The history of immunosuppression, as the patient has diabetes mellitus and autoimmune hemolytic anemia and is on corticosteroid therapy.
-
The diagnosis was confirmed by a positive galactomannan test and the marvelous response to voriconazole.
Chest x-ray AP view and abdominal ultrasonography were non-revealing (Fig. 1).
Noncontrast multislice CT scan of the brain shows atrophic brain changes (Fig. 2).
The patient was initiated with antibiotics including ceftriaxone, vancomycin, acyclovir, prednisolone, fluids, and dehydrating measures as well as control of blood sugar and blood pressure.
On follow-up, there was no improvement over the next few days, and the fever persisted despite the antibiotics and antiviral therapy.
MRI brain with contrast revealed
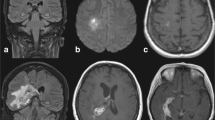
Multiple variable-sized scattered cerebral lesions at subcortical and deep parenchymal in the left thalamic, brain stem, and cerebellar focal lesions with signal pattern and necrotic cores showing restrictive diffusion, the largest measuring 2.5 cm at the left parietal region, subcortical in location. These lesions show no appreciable enhancement in the post-contrast series (Figs. 3 and 4), a picture suggestive of a multifocal inflammatory lesion. Based on the clinical background of being an immunocompromised patient, opportunistic brain infection mostly fungal is considered for clinical, laboratory correlation, and follow-up.
Aciclovir was discontinued and intravenous voriconazole was initiated after establishing the diagnosis with positive aspergillus galactomannan antigen assay in serum and CSF. The dose was started at 6 mg/kg twice daily on day one, followed by 4 mg/kg twice daily thereafter. The CSF for fungal culture proved to be negative after 4 weeks, the treatment was continued and the patient started to show gradual clinical improvement.
Follow-up of the patient was excellent, the patient regained consciousness and became afebrile, and the general state improved with residual weakness in both lower limbs.
Follow-up MRI brain with contrast after 2 weeks of treatment: the brain lesions decreased in size, and overall regressive course regarding the size of the supra and infratentorial multiple space-occupying lesions currently showing marginal contrast enhancement (Fig. 5).
Follow-up CSF after 4 weeks revealed clear CSF, cells 20/cc, neutrophils 40%, lymphocytes 60%, glucose 122 mg/dl, and proteins 42 mg/dl.
Discussion
Central nervous system aspergillosis is a highly fatal disease; it is the most common organ manifestation of disseminated invasive infection, usually via the bloodstream from a pulmonary focus [4].
Direct invasion of the brain from adjacent foci of aspergillosis in paranasal sinuses or the middle ear is a rare alternative route of infection [5, 6]. The source of infection in this patient may have been a small focus in the paranasal sinuses with infection reaching the brain via direct extension or the hematogenous route. The mortality rate of cerebral aspergillosis approaches 100% in these immunocompromised patients [7], but there are occasional case reports noting survival with combined aggressive antifungal therapy and surgical resection. This makes early diagnosis essential [8].
Cerebrospinal aspergillosis typically presents with a fever unresponsive to antibacterial or antiviral treatment and may cause headache, meningeal irritation, nausea vomiting, focal neurologic deficits, seizures, mental retardation, or lethargy [9,10,11].
The diagnosis of intracerebral aspergillosis was suggested from the history of immunosuppression state as the patient having diabetes mellitus and autoimmune hemolytic anemia and on corticosteroid therapy.
MRI was suggestive by the multiple variable-sized lesions with no enhancement, and the diagnosis was confirmed by a positive galactomannan test and the marvelous response to voriconazole.
The lack of contrast enhancement is typical of severely immunosuppressed patients suggesting the absence of an inflammatory response [12].
The clinical signs of spastic paraparesis with hyperreflexia and positive Babinisk’s sign localize a lesion to the spinal cord. The bladder is usually involved in spinal cord disease but the absence of bladder symptoms does not rule out spinal cord involvement [13]. A variety of presenting symptoms such as nasal stuffiness, headache, periorbital pain, diplopia, anosmia, lethargy, convulsions, and impaired consciousness have been described.
The diagnosis of cerebral aspergillosis is difficult because the presenting symptoms are not specific (stroke-like symptoms or seizures) [14], and fever may be absent [15]. Moreover, the pulmonary infection may not be present, cerebrospinal fluid findings are usually minimally abnormal with a slightly elevated protein level, and the organism is rarely cultured from the CSF [16, 17]. Furthermore, the lack of specific radiologic features and the relative rarity of this disease make cerebral aspergillosis less recognizable resulting in a delayed diagnosis and treatment [18].
Aspergillus abscesses usually occur in the presence of predisposing factors, e.g., diabetes, long-term use of glucocorticoids, transplanted patients, and malignant neoplasms [19, 20].
The galactomannan test is primarily targeted for early diagnosis of invasive aspergillosis; its use is recommended by the Infectious Diseases Society of America (IDSA) [21].
Galactomannan (GM) in CSF showed a good diagnostic performance; using GM in CSF, cerebral aspergillosis can be diagnosed or virtually ruled out without the need for a cerebral biopsy [22].
Voriconazole is now recognized as the first choice agent for invasive aspergillosis by the Infectious Diseases Society of America, and there are several reports supporting its efficacy in cerebral aspergillosis [21].
Prognosis is largely dependent on early diagnosis, the extent of invasion, and the host’s immune status [23]. Duration of antifungal treatment is not clearly established and typically continues for several months depending on the clinical and radiological response and ongoing immunosuppression [23].
The major reason for the devastating prognosis of CNS aspergillosis is the poor penetration of antifungal drugs into the CNS, with the exception of voriconazole [24].
Most antifungal agents are large molecules which makes sufficient penetration into the central nervous system unlikely. Concentrations of voriconazole exceeding the inhibitory concentrations for aspergillus species were found repeatedly in cerebrospinal fluid and brain tissue, including brain abscess material; voriconazole is the smallest molecule with activity against aspergillus species [25].
The patient was discharged home after 4 weeks on oral voriconazole 200 mg twice daily, for long-term treatment and indefinite duration, physiotherapy, and neurological follow-up because of wasting and decreased motor power in both lower limbs, together with some cerebellar manifestations.
Follow-up monthly for 1 year showed his progressive improvement with only some weakness in both lower limbs.
Diagnosis
Acute invasive aspergillosis is the diagnosis in a diabetic patient with autoimmune hemolytic anemia.
Conclusion
Aspergillosis should be suspected in any immunocompromised patient with neurological symptoms and signs when acute in onset. Central nervous system aspergillosis is managed most commonly with voriconazole as a single antifungal drug therapy, and most do not require surgical intervention. Once there is evidence of infection as indicated by a positive galactomannan antigen test or suspicious radiographic findings, antifungal therapy (most often voriconazole) is administered to prevent the progression of the disease into invasive disease; the duration of antifungal prophylaxis should be individualized based on the clinical status of the patient.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CNS:
-
Central nervous system
- MRI:
-
Magnetic resonance imaging
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- GCS:
-
Glasgow Coma Scale
- ECG:
-
Electrocardiography
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase myoglobin binding
- ANA:
-
Anti-nuclear antibody
- CRP:
-
C-reactive protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ESR:
-
Erythrocyte sedimentation rate
References
Lin SJ, Schranz J, Teutsch SM (2001) Aspergillosis case-fatality rate: a systematic review of the literature. Clin Infect Dis 32:358–66
Lutsar I, Roffey S, Troke P (2003) Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis 37:728–32
Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E (2005) Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106(8):2641–5. https://doi.org/10.1182/blood-2005-02-0733.
Pagano L, Ricci P, Montillo M, Cenacchi A, Nosari A, Tonso A, Cudillo L, Chierichini A, Savignano C, Buelli M, Melillo L, La Barbera EO, Sica S (2005) sing voriconazole treatment. Blood 106:2641–5
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A, Vehreschild MJGT, Viscoli C, Cornely OA (2018) Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. https://doi.org/10.1016/j.cmi.2018.01.002
Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE (2016) Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. https://doi.org/10.1093/cid/ciw326
Epstein NE, Hollingsworth R, Black K, Farmer P (1991) Fungal brain abscesses (aspergillosis/mucormycosis) in two immunosuppressed patients. Surg Neurol 35:286–289
Miaux Y, Singer B, Leder S, Guermazi A, Bourrier P (1994) MR imaging of cerebral aspergillosis: different patterns in the same patient. AJNR Am J Neuroradiol 15:1193–1195
Walsh TJ, Hier DB, Caplan LR (1985) Aspergillosis of the central nervous system: clinicopathological analysis of 17 patients. Ann Neurol 18:574582
Jantunen E, Volin L, Salonen O et al (2003) Central nervous system aspergillosis in allogeneic stem cell transplant recipients. Bone Marrow Transplant 31:191196
Hagensee ME, Bauwens JE, Kjos B, Bowden RA (1994) Brain abscess following marrow transplantation: experience at the Fred Hutchinson Cancer Research Center, 19841992. Clin Infect Dis 19:402408
Ruhnke M, Kofla G, Otto K, Schwartz S (2007) CNS Aspergillosis Recognition, Diagnosis and Management. CNS Drugs 21:659–676
David BT, Steward O (2010) Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp Neurol 226(1):128–35. https://doi.org/10.1016/j.expneurol.2010.08.014. Epub 2010 Aug 14. PMID: 20713043; PMCID: PMC2955760
Beal MF, O’Carroll CP, Kleinman GM, Grossman RI (1982) Aspergillosis of the nervous system. Neurology 32:473–479
Lyons RW, Andriole VT (1986) Fungal infections of the CNS. Neurol Clin 4:159–170
Cohen J (1991) Clinical manifestations and management of aspergillosis in the compromised patient. In: Warnock DW, Richardson MD (eds) Fungal Infection in the Compromised Patient. John Wiley and Sons, Chichester, pp 117–151
Lyons RW, Andriole VT (1986) Fungal infections of the CNS. Neurol Clin 4:159–170
Pushker N, Meel R, Kashyap S, Bajaj MS, Sen S (2011) Invasive aspergillosis of orbit in immunocompetent patients: treatment and outcome. Ophthalmology 118:1886–1891
Spapen H, Spapen J, Taccone FS, Meersseman W, Rello J, Dimopoulos G et al (2014) Cerebral aspergillosis in adult critically ill patients: a descriptive report of 10 patients from the AspICU cohort. Int J Antimicrob 43(2):165e9
Yan X, Zong F, Zhao X, Sun K, Jin L, Wang J et al (2014) Analysis of pathogenic spectrum and risk factors in 165 non-transplant patients with invasive fungal disease. Zhonghua Jie He He Hu Xi Za Zhi 37(7):487e91
JE. (2016) Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:e1–e60. https://doi.org/10.1093/cid/ciw326
Chong GM, Maertens JA, Lagrou K, Driessen GJ, Cornelissen JJ, Rijnders BJ (2016) Diagnostic Performance of Galactomannan Antigen Testing in Cerebrospinal Fluid. J Clin Microbiol. 54(2):428–31. https://doi.org/10.1128/JCM.02913-15. Epub 2015 Dec 9. PMID: 26659218; PMCID: PMC4733214
Reinwald M, Spiess B, Heinz WJ, Heussel CP, Bertz H, Cornely OA, Hahn J, Lehrnbecher T, Kiehl M, Laws HJ, Wolf HH, Schwerdtfeger R, Schultheis B, Burchardt A, Klein M, Dürken M, Claus B, Schlegel F, Hummel M, Hofmann WK, Buchheidt D (2013) Aspergillus PCR-based investigation of fresh tissue and effusion samples in patients with suspected invasive Aspergillosis enhances diagnostic capabilities. J Clin Microbiol 51(12):4178–85. https://doi.org/10.1128/JCM.02387-13. Epub 2013 Oct 9. PMID: 24108612; PMCID: PMC3838048
Schwartz S, Thiel E (2004) Update on the treatment of cerebral aspergillosis. Ann Hematol 83(Suppl 1):S42–S44. https://doi.org/10.1007/s00277-004-0849-8. PMID: 15124667
Schwartz S, Thiel E (2009) Cerebral aspergillosis: tissue penetration is the key. Med Mycol 47(Suppl 1):S387–S393. https://doi.org/10.1080/13693780802537953. Epub 2009 Feb 28 PMID: 19255905
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Hamdy Ibrahim was the contributor in writing the manuscript and collected the patient data. All authors took part in the realization and implantation of this work. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for this section.
Consent for publication
Available consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, H., Maksod, S.A., Khorshed, M. et al. Successful treatment of multiple intracerebral aspergillosis with voriconazole alone in an Egyptian diabetic patient with autoimmune hemolytic anemia. Egypt J Intern Med 35, 69 (2023). https://doi.org/10.1186/s43162-023-00254-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00254-9