Abstract
Background
Hepatitis C is associated with metabolic effects and fatty liver disease. The effect of different direct antivirals on the liver steatosis, and the metabolic profile, still needs to be established. The aim of this study is to determine the effect of achieving the sustained virological response after 12 weeks (SVR-12 weeks) with different combinations of direct antiviral drugs, on the hepatic steatosis, and fibrosis presented by laboratory and transient elastography parameters. Our study population is nondiabetic, chronically infected HCV Egyptian patients and naïve to any form of HCV treatment.
Methods
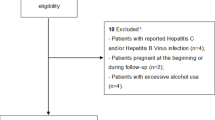
This cohort study was carried on 100 nondiabetic HCV treatment-naïve patients attending the Hepatology Clinic, in the Gastroenterology and Hepatology Department, Ain Shams University, and Kobry El Koba Military Hospital. The patients were divided into four groups according to their treatment regimens as follows: group A: 25 patients who received sofosbuvir (400 mg) and daclatasvir (60 mg) daily for 12 weeks; group B: 25 patients who received sofosbuvir (400 mg) and ledipasvir (90 mg) daily for 12 weeks; group C: 25 patients who received ombitasvir (12.5 mg), paritaprevir (75 mg), and ritonavir (50 mg) daily for 12 weeks; and group D: 25 patients who received sofosbuvir (400 mg) and simeprevir (150 mg) daily for 12 weeks. All patients were subjected to the following investigations: HCV quantitative PCR before and after 12 weeks of treatment, clinical and laboratory metabolic evaluation including alfa-fetoprotein level, thyroid profile assessment, ferritin level, pelvi-abdominal ultrasound, and FibroScan examination.
Results
All patients achieved SVR after 12 weeks. FibroScan median decreased (P < 0.001) from 19.29 ± 6.97 kPa at baseline to 14.15 ± 6.48 kPa at SVR12. NAFLD score median increased from 1.88 (1.49–2.22) at baseline to 2.01 (1.61–2.33) after 12 weeks of treatment. The highest level of NAFLD score was in group C, and the lowest was in group B. The BMI mean decreased from 28.31 ± 1.53 at baseline to 28.07 ± 1.52 at SVR12. HbA1C level mean decreased from 5.73 ± 0.23 at baseline to 5.40 ± 0.24 at SVR12. In addition, liver enzymes, cholesterol, triglycerides, APRI score (AST-platelet ratio index), and HBA1C decreased after 12-week treatment with a statistically significant difference, while the mean LDL increased after 12 weeks of treatment.
Conclusions
DAAs affect the metabolic profile of the treated patients. There is a noticed improvement in the FibroScan, NAFLD score, and lipid profile after achieving the SVR-12 weeks. However, LDL is increased after viral cure, mostly due to viral-host molecular interaction.
Similar content being viewed by others
Introduction
Egypt has the highest prevalence of hepatitis C virus infection worldwide (6.3% in 2015); 90% of the infected population are infected by genotype 4 [1]. The estimated number of patients needed to treat in Egypt to eliminate HCV infection by 2030 was calculated using a modeling study and found to be 350,000 patients per year [2]. The introduction of oral direct-acting antivirals (DAAs) is considered an important breakthrough in virology in the last decade. DAAs generated a great impact on the prognosis and quality of life of HCV patients [3].
The effect of HCV on the metabolic state is well established in the form of: steatosis, steatohepatitis, and hyperlipidemia. The effect of the DAAs on the metabolic profile following HCV cure is still under extensive research. Theoretically, the insulin resistance state caused by HCV infection should be reversed upon cure; however, the data is controversial [4,5,6]. Insulin resistance and diabetes are important risk factors in increasing the rapidity of progression of the chronic hepatitis to cirrhosis [7] and lowering the response to antiviral medications [8].
The most recent American Association for the Study of Liver Diseases (AASLD) [9] and European Association for the Study of the Liver (EASL) [10] guidelines have removed sofosbuvir and daclatasvir from the regimens used in HCV treatment. However, they are still the primary treatment in the Egyptian guidelines as stated by The National Committee for Control of Viral Hepatitis in Egypt due to their cost-effectiveness, efficacy, and safety in genotype 4.
Transient elastography results could be inconsistent across patients due to the presence of lower stages of fibrosis (F1–2), steatosis, or high BMI (body mass index) [11]. Thus, caution is advised when interpreting these populations, and further research is needed.
The aim of this study is to evaluate the effect of direct-acting antiviral drugs on hepatic steatosis, metabolic profile and scores, and transient elastography scan (FibroScan) parameters, in treating HCV-naïve chronically infected Egyptian patients, with normal baseline metabolic profile (nondiabetic and normo-lipidemic). Then, we evaluate the patients after 3 months of treatment by direct-acting antivirals and the effect of achieving their sustained virological response (SVR) on the metabolic profile and radiologic parameters.
Methods
This prospective cohort study was carried in the outpatient virology clinic specialized in treating HCV-infected patients (as a part of the National Campaign for HCV treatment) at the Gastroenterology and Hepatology Department, Ain Shams University, and at Kobry El-Kobba Military Hospital (Armed Forces Medical Complex Kobry El Qobba) between January 2018 and August 2019.
The study included 100 chronically infected HCV patients and naïve to HCV treatment. The patients were diagnosed as HCV infected through the national screening campaign by detecting HCV antibodies by ELISA. Further confirmation of active HCV infection is done by quantitative PCR for HCV RNA (COBAS TaqMan; Roche Diagnostic systems) with a lower limit of detection of 15 IU/ml.
This study protocol acquired the approval of the Ethical Committee of Faculty Medicine, Ain Shams University, Cairo, Egypt. Approval number of the protocol is FMASU-MD (322/2017). Informed written consent was taken from all the patients before inclusion in the study. All methods were carried out in accordance with Helsinki guidelines and regulations.
The exclusion criteria included the following:
-
1.
Patients with previous antiviral treatment of any type
-
2.
Patients with HBV or HIV co-infection
-
3.
Pregnant females
-
4.
Patients with any metabolic comorbidity like diabetes, hyperlipidemia, hypercholesterolemia, hyperthyroidism, or hypothyroidism
-
5.
Patients with any autoimmune disease including autoimmune hepatitis
-
6.
Patients with hemochromatosis, Wilson disease, or bilharziasis
-
7.
Patients with hepatocellular carcinoma or with past history of hepatocellular carcinoma
-
8.
Patients with body mass index greater than 28 kg/m2
-
9.
Patients with any history of drug intake known to cause hepatic steatosis (e.g., methotrexate, amiodarone, tamoxifen)
The inclusion criteria are as follows:
-
1.
Patients chronically infected with HCV with anti-HCV-positive antibodies and further confirmed by positive PCR for HCV RNA
-
2.
Patients > 18 years old and < 70 years old
-
3.
Patients with platelet count of > 100,000/mm3 and hemoglobin level of > 10 g/dl
-
4.
Patients with normal thyroid, lipid profile, and HbA1C level
We calculated the Child-Pugh score for each patient according to the established clinical formulas.
Patients were divided into 4 groups according to their treatment regimen for HCV as follows:
-
1.
Group A: Twenty-five patients who received sofosbuvir (400 mg) and daclatasvir (60 mg) daily for 12 weeks
-
2.
Group B: Twenty-five patients who received sofosbuvir (400 mg) and ledipasvir (90 mg) daily for 12 weeks
-
3.
Group C: Twenty-five patients who received ombitasvir (12.5 mg), paritaprevir (75 mg), and ritonavir (50 mg) daily for 12 weeks
-
4.
Group D: Twenty-five patients who received sofosbuvir (400 mg) and simeprevir (150 mg) daily for 12 weeks.
All patients are subjected to the following at baseline:
-
(A).
Proper history evaluation: To exclude other causes of chronic hepatitis, any associated comorbidities, drug history of any antiviral drugs, or any drugs that induce hepatic steatosis
-
(B).
Full clinical examination: General examination and local abdominal examination to assess the general signs of liver cell failure (jaundice, palmar erythema, spider nevi, bilateral pitting lower limb edema, ascites, hepatic encephalopathy, etc.), hepatomegaly, and splenomegaly. Other systems were thoroughly examined to exclude comorbidities.
-
(C).
Laboratory investigations are as follows:
-
1.
Routine laboratory investigations including complete blood picture, liver enzymes, liver function tests, kidney function tests, urine analysis, lipid profile, fasting, and 2-h postprandial blood glucose and HbA1C
-
2.
Viral markers including HCV Ab by ELISA, HBs Ag, and HIV antibody test
-
3.
HCV RNA by PCR × (COBAS TaqMan; Roche Diagnostic systems), with a lower limit of detection of 15 IU/ml
-
4.
Alpha-fetoprotein and ceruloplasmin levels in serum
-
5.
Thyroid profile (TSH — free T3 — free T4)
-
6.
Serum ferritin, anti-bilharzial antibody, and CPK were tested.
-
7.
Antinuclear antibodies (ANA) and smooth-muscle antibodies (SMAs)
-
8.
Pregnancy test for married female patients in the child-bearing period
-
(D).
Radiological investigations were as follows:
-
1.
Pelvi-abdominal ultrasound: To report any changes of liver echogenicity, or nodularity, signs of portal hypertension (splenomegaly, dilated portal vein, ascites, etc.), or hepatic focal lesions
-
2.
FibroScan: To assess the degree of hepatic fibrosis before starting treatment
Results are expressed in kilopascal (kPa) that corresponds to the manufacturer’s recommendations and to the median of 10 validated measurements. Liver stiffness values range from 2.5 to 75 kPa [11]. Ten valid measures of liver stiffness are indicated with at least 60% success rate and interquartile range < 30% of the median value with results expressed in kilopascals (KPa). When liver stiffness values range from 2.5 to 7 kPa, then the fibrosis is mild or absent, whereas when values area is above 13 kPa, then the cirrhosis is present [12].
-
(E).
Upper endoscopy
Upper endoscopy (Olympus endoscope LUCERA 260) was done to all patients with splenomegaly, or any sign of portal hypertension to exclude the presence of esophageal, or fundal varices.
-
(F).
PRI score is as follows:
AST to platelet ratio index score (APRI score) was calculated for the patients before starting treatment.
It is calculated as (AST/upper limit of normal range)/platelet count (10ˆ9/L) × 100 [13].
There are several optimal APRI cutoff values for prediction of cirrhosis presented in the literature. A lower threshold of 0.5 and a higher one of 1.5 have been suggested for the identification of significant fibrosis [14].
-
(G).
NAFLD fibrosis score
NAFLD fibrosis score was calculated for the patients before starting treatment.
The score was developed in 2007 [15], where the authors stated that hyperglycemia, albumin, age, body mass index (BMI), platelet count, and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio were the solitary predictors of advanced fibrosis.
After 12 weeks of treatment, all patients of the study were subjected to the following:
-
(A).
Proper clinical examination
-
(B).
Laboratory investigations are as follows:
1) Complete blood picture, liver enzymes, liver and kidney function tests, fasting and 2-h postprandial blood glucose, lipid profile thyroid profile, and HbA1C
2) HCV RNA by PCR (COBAS TaqMan; Roche Diagnostic systems), with a lower limit of detection of 15 IU/ml
3) Alpha-fetoprotein level in serum
-
(C).
Radiological investigations are as follows:
-
1.
Pelvi-abdominal ultrasound: To detect any changes of liver echogenicity, nodularity, or signs of portal hypertension (splenomegaly, dilated portal vein, ascites, etc.)
-
2.
FibroScan: To assess the degree of hepatic fibrosis after treatment
-
(D).
APRI score calculation
-
(E).
NAFLD fibrosis score calculation
Quantitative PCR for HCV RNA
Serum HCV RNA was measured using the COBAS TaqMan HCV Test v2.0 (Roche Molecular Systems, Pleasanton, CA, USA; lower limit of quantitation (LLOQ) of 15 IU/ml) at baseline, all subsequent study visits during treatment, and posttreatment at weeks 4, 12, and 24. Patients with confirmed HCV RNA < LLOQ at the end of the treatment and posttreatment visits continued until the subsequent posttreatment visits, unless there was a confirmed virologic relapse. On-treatment virologic failure was defined as follows: breakthrough, i.e., confirmed by HCV RNA > LLOQ after having previously had HCV RNA < LLOQ while on treatment; rebound, i.e., confirmed > 1-log10 IU/ml increase in HCV RNA while on treatment; or nonresponse, i.e., HCV RNA persistently > LLOQ through 8 weeks of treatment. Relapse was defined as confirmed HCV RNA > LLOQ during the posttreatment period having achieved HCV RNA < LLOQ at the end of the treatment.
Statistical methods
Data were collected, revised, coded, and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations, and ranges when their distribution found parametric and median with interquartile range (IQR) when their distribution found nonparametric. Also, qualitative variables were presented as number and percentages.
The comparison between groups with qualitative data was done by using chi-square test.
The comparison between two groups with quantitative data and parametric distribution was done by using independent t-test while for nonparametric data by using Mann-Whitney test.
The comparison between two paired groups with quantitative data and parametric distribution was done by using paired t-test while for nonparametric data by using Wilcoxon rank test.
The comparison between more than two groups with quantitative data and parametric distribution was done by using one-way ANOVA test, followed by post hoc analysis using LSD test, while for nonparametric data by using Kruskal-Wallis test, followed by post hoc analysis using Mann-Whitney test.
Spearman correlation coefficients were used to assess the correlation between two quantitative parameters in the same group.
The confidence interval was set at 95%, and the margin of error accepted was 5%. So, the P-value was considered significant according to the following:
-
P-value > 0.05: Nonsignificant
-
P-value < 0.05: Significant
-
P-value < 0.01: Highly significant
Results
Our study evaluated the effect of direct-acting antiviral drugs on hepatic steatosis in HCV chronically infected naïve Egyptian patients at baseline and after reaching 12 weeks. All the patients were males with a mean age of 50.99 ± 8.746. All the patients achieved SVR12 despite receiving different DAA regimens.
The AST, ALT, and platelets were higher in group B at the baseline than the rest of the groups (A, C, and D). The mean PLT count was decreased from baseline upon reaching SVR12. There was difference in the mean PLT count across the four groups; the highest mean PLT count was in group B 159.68 ± 41.72, and the lowest was in group D 123.92 ± 16.01 (Fig. 1).
Liver enzymes (AST and ALT) decreased overtime and upon reaching SVR, where the mean AST was significantly decreased from 58.85±31.85 to 27.57± 9.90 (Table 1). In addition, the mean ALT levels were decreased from 53.54±28.39 at baseline to 23.96±8.92 at SVR12, reflecting a decreased necro-inflammatory activity after treatment. Also, there was a difference in the AST and ALT levels across the four studied groups at SVR12; the highest was in group B, and the lowest was in group D. The levels of serum albumin increased, the total bilirubin decreased, and the AFP decreased upon reaching the SVR12, when compared to the baseline (Tables 1 and 2).
The mean serum TG was significantly decreased from 101.68± 29.85 to 88.64±26.53 from baseline to SVR12 (Table 2, Fig. 2). The mean TG was different across the studied groups at SVR12, where the highest was in group D 102.36 ± 24.24 and the lowest was in group A 77.64 ± 25.37 (Fig. 3). The mean serum cholesterol was significantly decreased on comparing all the patients before starting treatment, and at SVR-12, the four groups were similar in their SVR12 cholesterol levels (Fig. 2). The mean HDL was significantly increased from 36.15±9.63 at baseline to 48.97 ±9.86 at SVR12 (Figs. 2 and 3, and Table 2). Moreover, there was a difference in HDL level between the four studied groups at SVR12, where the highest was in group D with a mean of 52.68 ±7.78 and the lowest was in group B with a mean of 45.84 ± 9.85 (Figs. 2 and 3). The mean LDL was significantly increased from 81.79±21.43 to 88.09 ±21.80, from baseline to SVR12, while there was no difference between the four groups at SVR-12 (Fig. 2).
APRI score mean was significantly decreased, while NAFLD score (Fig. 4) was increased from baseline to SVR12 (Fig. 5).
There was no difference in the FibroScan score, on comparing all the patients, before starting treatment, and at SVR 12. However, FibroScan median decreased from 19.29± 6.97 kPa at baseline to 14.15±6.48 kPa at SVR12. The FibroScan median at SVR12 was similar across the four groups (Fig. 5).
BMI mean decreased from 28.31 ± 1.53 at baseline to 28.07 ± 1.52 at SVR12 (Table 1). In addition, HbA1C level mean decreased from 5.73±0.23 at baseline to 5.40 ± 0.24 at SVR12 (Table 2). There was also a difference in HbA1C level across the studied groups at SVR12, where the highest level was in group D with a mean of 5.52 ± 0.23 while the lowest level was in group B with a mean of 5.34 ± 0.25.
Discussion
After the high success of the combination regimens across the various studies and treatment centers, the AASLD guidelines issued a major update in November 2019 regarding HCV management [9]. In addition, the WHO plan to eliminate HCV globally by 2030 is working with high efficacy [16]. The HCV burden in Egypt is high. A study in our Ain Shams University published recently showed that the prevalence of HCV antibodies in healthcare workers was 8%, while among the attending patients was 19.8%. The highest prevalence was among patients > 50 years of age or living outside Cairo [17].
All patients in our study achieved SVR-12. This success rate is similar to many studies done after the introduction of DAAs [18,19,20]. Our study population is selected with a normal baseline metabolic profile, despite being chronically infected by HCV. Thus, any change in their metabolic profile post-therapy is attributed to the effect of medications and the HCV cure.
Our study shows that the efficacy of the different DAAs is comparable. The achievement of viral elimination and SVR was associated with an improvement in the blood and metabolic indices, along with an improvement in the prognostic scores as the APRI and the NAFLD scores. Moreover, the SVR is associated with a better transient elastography parameters posttreatment, indicating stabilization of the patient’s condition, and possible regression of the liver fibrosis stage.
The use of APRI and FIB-4 scores was compared to transient elastography. It was found that the combination of APRI/FIB-4 highly predicts the significant fibrosis F3/F4, while the FIB-4 alone highly predicts the cirrhosis F4 [14]. In our study, the APRI score was decreased, and NAFLD score was increased after reaching SVR-12 weeks.
The elevation in the lipid profile especially LDL could be explained by HCV nonstructural protein 5A activation of the sterol regulatory element-binding protein-1c (SREBP-1c), responsible for the activation of the lipogenic genes [21].
HCV is known to cause steatosis generally; however, the severity of steatosis is related to the viral genotype. Genotype 3 is most commonly associated with an increase in the steatosis severity, as the phenylalanine residue (position 164) of the HCV core particle causes an enhanced accumulation of lipid droplets in the hepatocytes [22].
In addition, HCV-associated steatosis could be caused by host factors as BMI, uncontrolled blood sugar, diabetes, and hyperlipidemia. Moreover, these host factors could participate in the increased progression to fibrosis and cirrhosis [23]. In our study, the fasting blood sugar and BMI were decreased upon achieving SVR.
In a previous Egyptian study using the regimen daclatasvir-sofosbuvir for 3 months, the total cholesterol was increased upon achieving SVR-12 and SVR-24, along with an increase in the LDL levels, but the triglycerides remained unchanged. This could be attributed to the baseline metabolic state of their cohort, as two-thirds of the included patients had a hyperlipidemic state prior to treatment [4]. Another study, in our Ain Shams virology treatment center, done on HCV-infected patients with decreased eGFR (of 30 ml/min or less), they found that after achieving SVR, the triglycerides levels, and HbA1C, were decreased, while the total cholesterol, LDL, and HDL levels were increased [18].
In our study (as shown in Fig. 2), we chose patients with normal metabolic profile and excluded the hyperlipidemic and the diabetic HCV-infected patients. We found that the decline upon achieving SVR-12 weeks was in the levels of HBA1C, the fasting blood glucose, the triglycerides, and the total cholesterol, while the level of HDL was elevated. This indicates an improvement in their metabolic profile; however, their LDL levels were increased. Our results agree with Gitto et al., where they observed worsening of the cholesterol, and LDL level, along with improved HDL after DAAs treatment, on a cohort of 100 of treated HCV patients [6].
Hashimoto et al. explained this disturbance in LDL by the deregulation of the lipid metabolism with subsequent dropping of the lipid droplet production and rebound elevation in the circulating LDL following the rapid viral suppression by the DAAs [24]. In addition, it is proved that the inflammatory state does not resolve completely even after the virus is completely eliminated by the DAAs [25]. Residual histological steatosis post-SVR could be detected on short-term follow-ups, despite the normalization of the liver enzymes [5].
It is controversial if there is a fibrosis regression post SVR-12; however, most of the fibrosis non-invasive parameters tend to decrease after cure of the viral load. It is shown that liver stiffness is influenced not only by the degree of liver fibrosis but also by necro-inflammatory activity [20]. This could explain the small decline in the transient elastography parameters in our study. In our study, liver stiffness decreased over time, and there were significant differences between baseline and SVR-12 weeks among the studied groups.
Conclusion
DAAs affect the metabolic profile of the treated HCV patients. Different regimens may have different effects on the metabolic profile and the liver stiffness in HCV-treated patients. Early DAAs are still used in Egypt with good efficacy and good metabolic outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request. All figures supplied are original figures.
Abbreviations
- AASLD:
-
The American Association for the Study of Liver Diseases
- ALT:
-
Alanine transaminase
- APRI score:
-
AST to platelet ratio index score
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- DAAs:
-
Direct-acting antivirals
- EASL:
-
The European Association for the Study of the Liver
- ELISA:
-
The enzyme-linked immunosorbent assay
- HBA1C:
-
Hemoglobin A1C
- HCV:
-
Hepatitis C virus
- HDL:
-
High-density lipoprotein
- HIV:
-
Human immunodeficiency virus
- kPa:
-
Kilopascal
- LDL:
-
Low-density lipoprotein
- LLOQ:
-
Lower limit of quantitation
- PCR:
-
Polymerase chain reaction
- PLT:
-
Platelets
- RNA:
-
Ribonucleic acid
- SVR:
-
Sustained virological response
References
Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH et al (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2(3):161–176
Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I (2017) Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 9:17–25
Chae HB, Park SM, Youn SJ (2013) Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. Sci World J 2013:704912
Eltebi D, Mohamed S, Ammar I (2019) Metabolic changes in Egyptian patients with HCV related chronic liver disease after oral antiviral therapy. J Infect Dis Ther 7(392):1–5
Enomoto M, Ikura Y, Tamori A, Kozuka R, Motoyama H, Kawamura E et al (2018) Short-term histological evaluations after achieving a sustained virologic response to direct-acting antiviral treatment for chronic hepatitis C. United European Gastroenterol J 6(9):1391–1400
Gitto S, Cicero AFG, Loggi E, Giovannini M, Conti F, Grandini E et al (2018) Worsening of serum lipid profile after direct acting antiviral treatment. Ann Hepatol 17(1):64–75
Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP et al (2008) Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 134(2):416–423
Khattab M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L (2010) Insulin resistance predicts rapid virologic response to peginterferon/ribavirin combination therapy in hepatitis C genotype 4 patients. Am J Gastroenterol 105(9):1970–1977
Ghany MG, Morgan TR, Panel A-IHCG (2020) Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases–infectious diseases society of america recommendations for testing, managing, and treating hepatitis c virus infection. Hepatology. 71(2):686–721
Pawlotsky J-M, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G et al (2020) EASL recommendations on treatment of hepatitis C: final update of the series<sup>☆</sup>. J Hepatol 73(5):1170–1218
Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G et al (2007) Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 56(7):968–973
Castera L (2012) Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 142(6):1293–302.e4
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38(2):518–526
Papadopoulos N, Vasileiadi S, Papavdi M, Sveroni E, Antonakaki P, Dellaporta E et al (2019) Liver fibrosis staging with combination of APRI and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann Gastroenterol 32(5):498–503
Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC et al (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45(4):846–854
Organization. WH (2016) Global health sector strategy on viral hepatitis 2016-2021: towards ending viral hepatitis. geneva: who document and production services, pp 1–56
Anwar WA, El Gaafary M, Girgis SA, Rafik M, Hussein WM, Sos D et al (2021) Hepatitis C virus infection and risk factors among patients and health-care workers of Ain Shams University hospitals, Cairo, Egypt. PLoS One 16(2):e0246836
Allam AS, Elmeged MLA, Ghaly SM, Ahmed OA, Naguib GG, Abohalima AS (2022) Impact of direct-acting antiviral therapy on metabolic profiles and adiponectin serum level in different categories of patients with chronic hepatitis C infection. Egypt Liver J 12(1):32
Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA (2018) Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol 24(11):1269–1277
Asselah T, Marcellin P, Schinazi RF (2018) Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int 38 Suppl 1(Suppl 1):7–13
Xiang Z, Qiao L, Zhou Y, Babiuk LA, Liu Q (2010) Hepatitis C virus nonstructural protein-5A activates sterol regulatory element-binding protein-1c through transcription factor Sp1. Biochem Biophys Res Commun 402(3):549–553
Hourioux C, Patient R, Morin A, Blanchard E, Moreau A, Trassard S et al (2007) The genotype 3-specific hepatitis C virus core protein residue phenylalanine 164 increases steatosis in an in vitro cellular model. Gut 56(9):1302–1308
Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A et al (1999) Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology 29(4):1215–1219
Hashimoto S, Yatsuhashi H, Abiru S, Yamasaki K, Komori A, Nagaoka S et al (2016) Rapid increase in serum low-density lipoprotein cholesterol concentration during hepatitis C interferon-free treatment. PLoS One 11(9):e0163644
Vranjkovic A, Deonarine F, Kaka S, Angel JB, Cooper CL, Crawley AM (2019) Direct-Acting Antiviral Treatment of HCV Infection Does Not Resolve the Dysfunction of Circulating CD8+ T-Cells in Advanced Liver Disease. Front Immunol 10:1926. https://doi.org/10.3389/fimmu.2019.01926
Acknowledgements
Mohamed El-Nakeep MSc. for conducting statistical analysis
Funding
All authors declare they received no funding on conducting the study.
Author information
Authors and Affiliations
Contributions
AEG: Formulation of the research idea and clinical evaluation of the patients. TY: Formulation of the research idea and supervision of the work. WI: Formulation of the research idea and supervision of the data collection. HAA: Laboratory assessment of the cases and writing of the manuscript. SAeB: Laboratory assessment of the cases. MW: Collection of the patients’ data and writing of the manuscript. SEN: Formulation of the research idea, clinical evaluation of the patients, and writing of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol had the ethical approval of the Ethical Committee of Faculty Medicine, Ain Shams University, Cairo, Egypt. Approval number of the protocol is FMASU-MD (322/2017). Informed written consent was taken from all the patients before inclusion in the study. All methods were carried out in accordance with Helsinki guidelines and regulations. (We can provide a copy of the original document.)
Consent for publication
Not applicable, as no individual or personal data were used in this study. All authors consent on publication of the submitted work.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Ghandour, A., Youssif, T., Ibrahim, W. et al. The effect of different direct antivirals on hepatic steatosis in nondiabetic and naïve hepatitis C-infected Egyptian patients. Egypt J Intern Med 35, 12 (2023). https://doi.org/10.1186/s43162-023-00197-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-023-00197-1