Abstract
Background
Pelvic floor dysfunction (PFD) is a global health problem affecting millions of women worldwide. Vaginal childbirth has been reported to be the most important factor in the etiology of PFD though a prior study also reported a high prevalence of PFD in nulliparous women. Some previous studies had suggested Urinary incontinence before pregnancy as a major risk factor for incontinence later in life, thus prevention of PFD has become a major priority in women’s health, and identification of women at risk is a key element in current prevention strategies It is therefore necessary to investigate the prevalence and risk factors for PFD in Nulligravida women who have never been pregnant to enable preventative measures especially as it regards lifestyle modification.
Methods
Participants were 160 Nulligravida students aged between 17 and 26 years. They were screened for pelvic floor dysfunction using an Australian pelvic floor questionnaire which assessed their bladder function, bowel function, pelvic organ prolapse, and sexual function. Descriptive statistics of frequency and percentage were used to summarize categorical variables. Univariate analysis of Fisher’s exact test and Wilcoxon rank-sum test were conducted to show the association of categorical and continuous variables with pelvic floor dysfunction (PFD) respectively. The risk factors of PFD with p values < 0.05 were considered significant. All analyses were performed using R Statistical Computing Programming version 4.2.2.
Results
The prevalence of having at least one of any of the PFD was 73.1% while the prevalence of bladder, bowel, prolapse, and sex dysfunction were 25.63% (41/160), 53.75% (86/160), 1.88% (3/160), and 23.13% (37/160) respectively. BMI was not a significant risk factor for any type of PFD while multi-variable logistic regression identified the level of study, height, history of UTI, and non-sport participation as significant (p < 0.05) risk factors for at least one type of PFD with Odd ratio of 4.91, > 100, 8.47, and 2.86 respectively.
Conclusion
There is high prevalence of PFD among Nulligravida students, with non-participation in sports and history of urinary tract infections being the main significant risk factors.
Similar content being viewed by others
Introduction
Pelvic floor dysfunction (PFD) is a global health problem affecting millions of women worldwide and comprises a broad range of clinical dysfunctions such as urinary incontinence (UI), fecal incontinence (FI), pelvic organ prolapse (POP) vaginal laxity (VL), vaginal wind (VW), and overactive bladder (OAB). Also inclusive are sensory or emptying abnormalities of the lower urinary tract, defecation dysfunction, and sexual dysfunction (SD) [1, 2]. These symptoms lead to a broad range of quality-of-life impairments, from minor discomfort and embarrassment to severe damage to function and quality of life [3]. Adult women may have one or more of these dysfunction, which adversely affects the quality of life, including sexual health, functional status, social constraints, and psychological well-being [4]. Global reports of POP prevalence vary greatly, ranging from 3 to 50% [5].
Though vaginal childbirth has been reported to be the most important factor in the etiology of PFD [6], a prior study reported a high rate of more than one type of PFD in nulliparous women with very common clinically significant symptoms and associated bother [1]. Other major risk factors for subsequent pelvic dysfunction are UI before pregnancy, ethnicity, age at birth of the first child, BMI, family history of PFD (mother and sister), baby’s weight [6], and maternal height (if < 160 cm and baby > 4 kg) [7]. The association of overweight and obesity with stress incontinence has been shown in a previous study [8]. However, in the Nurses’ Health study, increased waist circumference but not BMI predicted incident stress urinary incontinence (SUI) among middle-aged women [9]. Therefore, cross-sectional studies show that in addition to BMI, waist-hip ratio and thus abdominal obesity may be an independent risk factor for incontinence in women. Obesity is considered an important independent risk factor for pelvic floor dysfunction, and a strong association between obesity and urinary incontinence in women has been clearly documented [10]. Obesity has also been associated with fecal incontinence, which affects 16–68% of obese individuals [11]. The prevalence of fecal incontinence has also been shown to decrease after weight loss following bariatric surgery, further emphasizing the importance of obesity as a modifiable risk factor.
Physical activity has numerous health benefits and women are encouraged to be active, however, there may be a threshold at which physical activity’s benefit for the pelvic floor is counterproductive. Mild or moderate activity and strenuous activity may impact pelvic floor disorders differently and in a bidirectional manner. Regular low-impact activity, like walking, is associated with a lower prevalence of stress incontinence [12]. However, many young women report stress urinary incontinence during high-impact, vigorous-intensity activities: 28% of college varsity athletes, 41% of elite athletes, and 43% of women participating in club sports [13].
Pelvic floor disorders are common, costly, and distressing conditions for women [13] and a prior study reported that 12.8% of women who had never given birth experienced pelvic floor dysfunction [14]. It has also been suggested from a prior study that one of the major risk factors for subsequent pelvic dysfunction is UI before pregnancy [15]. Therefore, lifestyle changes with behavioral modifications that can prevent PFD need to be adopted from an early phase of life. Prior evidence would suggest that in some cases incontinence later in life can be prevented or the onset delayed with primary prevention and actions taken early, even in the absence of incontinence [15].
Therefore, an investigation of the prevalence and risk factors of PFD in Nulligravida women who have never been pregnant will give an insight into the risks in these young women in order to create awareness and also offer preventative measures especially as it regards lifestyle modification. This will greatly minimize the risks of this population of women developing PFD later in life. This is important as a previous study suggested that the identification and standardization of pelvic floor muscles (PFM) strength in nulliparous women of different ages can help predict urinary and fecal incontinence as well as sexual dysfunction in a woman’s first pregnancy [16].
Materials and methods
Participants selection
One hundred and sixty unmarried nulligravida female students of the College of Medicine, University of Lagos not less than 18 years participated in this study. Excluded were students who had experienced a pregnancy or undergone any form of abdominal surgery prior to the time of the study.
Study design
This was a cross-sectional survey with participants recruited by convenience. Though the sample size was calculated to be 152 [17], 160 participants were recruited due to the availability of financial resources and time.
Sample size calculation
n = desired sample size
Z = the standard normal variate usually set at 1.96 (population critical value).
P = expected prevalence rate for the population based on previous studies or pilot study = 0.128.
d = absolute error.
n = (1.96)2 × (0.128) × (0.872)/0.052.
n = 0.429/0.0025 = 171.5
nf = n/[1 + n/N].
nf = minimum sample size for population < 10,000.
n = minimum sample size for population > 10,000.
N = estimated population of female students in college of medicine, which is estimated to be 1381.
nf = 171.5/[1 + 171.5/1381].
nf = 171.5/ (1 + 0.124) = 171.5/1.124.
nf = 152.
Sample size for study = 152.
Materials
The instrument used for the collection of data was the Australian Pelvic Questionnaire that collected information on all four types of PFD. The questionnaire consists of four sections, which cover all four types of PFD. Each section contains 10–15 questions. All answers were graded from 0 to 3, where zero means no symptom present and 3 means the most frequent or severe symptom. Additionally, each section has a question about the grade of bother due to symptoms, rated 0–3 (0 indicates no bother and 3 indicates severe disturbance). All questions from each section can be logically divided into primary symptoms, which are mandatory to diagnose a condition, and secondary symptoms, giving extra information on the severity of primary symptoms, such as reduced fluid intake, pad usage, laxative use, and bother. For analysis, primary symptoms from the questionnaire were selected according to International Continence Society (ICS) definitions for various types of fecal (FD) or urinary (UD) dysfunction. This study aimed to comprehensively investigate all possible types of symptoms and dysfunctions besides incontinence symptoms commonly described in other studies It investigated more questions from the questionnaire related to symptoms of overactive bladder in the urinary section and obstructed defecation in the FD section. For the sexual section, dyspareunia and vaginal laxity and tightness were used as primary symptoms. Regarding prolapse, all questions included in that section can be regarded as primary symptoms of prolapse. These symptoms were selected as primary because they were included in the Pelvic Floor Distress Inventory and repeatedly utilized in previous studies [1, 18]. The questionnaire additionally contains a total section score for UD, FD, POP, and sexual dysfunction (SD). It was calculated by adding all individual symptom scores in each section. In this analysis, symptoms with grade 2 or 3 (meaning symptoms present at least once weekly) in the questionnaire were considered clinically significant symptoms. The Australian Pelvic Questionnaire has a Cronbach’s alpha of 0.7 for all the subscales of the questionnaire and Kappa coefficients of agreement for the test–retest analyses varied from 0.5 to 1.0 [19]
Ethical consideration
Ethical approval for this study was sought and obtained from the institutional Health Research and Ethics Committee with approval number: CMUL/HREC/08/17/226. The purpose, relevance, and significance of the study were explained to the participants and their informed consent was obtained.
Research protocol
Participants were recruited by convenience and the demographic data was obtained to screen out those who did not meet the inclusion criteria. A total of 200 structured copies of the questionnaire were distributed and 179 were returned with one hundred and sixty (160) valid for analysis. Prior to the distribution of the questionnaire, the aim and objectives of the study were clearly explained to the participants. Participants’ approval was sought, after which the questionnaires were distributed by hand to the participants. The questionnaire was self-administered. Participants were assured of the confidentiality and anonymity of their data. The questionnaire was then collected upon completion.
Data analysis
Descriptive statistics of frequency and percentage were used to summarize categorical variables. The parametric distribution of continuous variables was assessed using the Shapiro–Wilk normality test. The median and interquartile range were used to describe the continuous variables. Univariate analysis of Pearson’s chi-square test, Fisher’s exact test, and Wilcoxon rank-sum test were conducted to show the association of categorical and continuous variables with pelvic floor dysfunction (PFD) respectively. The factors that achieved a p value of ≤ 0.2 were enrolled in multi-variable backward logistic regression analysis to determine the significant risk factors of PFD. In the determination of PFD, score ≥ 1 was considered as PFD and score < 1 was assigned non-PFD. The risk factors of PFD with p values < 0.05 were considered significant. All analyses were performed using R Statistical Computing Programming version 4.2.2.
Results
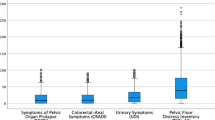
One hundred and sixty university female students aged from 17 to 26 years old were included in this study. The median age was 21 years and Yoruba was the majority ethnic group (72%). Fifty-three (33%) of the participants were in their fourth year at the university and significantly associated with PFD (Table 1). The overall median height, weight, and BMI were 1.65 m, 57 kg, and 21.4 kg/m2 respectively. The height of non-PFD and PFD were statistically different (p = 0.001) (Table 1). Predominantly were normal BMI (76%) and age group 20–24 years (68%), Table 2. The overall 32 (20%) of the students had a history of UTI of which 29 (25%) were found to be associated with any PFD (p = 0.013). Most of the students had no history of family PFD (96%). The students’ sports involvement 59 (37%), types of sport, and frequency of sports activities were associated with PFD (p < 0.05) (Table 3). The percentages and associated categorical variables with bladder, bowel, prolapse, and sexual dysfunction were shown in Table 4. Similarly, the continuous variables associated with the four types of PFD are displayed in Fig. 1. The symptoms of bladder dysfunction of nocturia, urge, and urge incontinence (UI) had scores ≥ 1 of 36.87%, 39.87%, and 38.75% respectively. The symptoms of bowel dysfunction of flatal incontinence (FLI) and obstructed defecation (OD) also had scores ≥ 1 of 30.62% and 49.25%. The Prolapse symptoms and symptoms of sexual dysfunction were less than 10% (Fig. 2). The prevalence of bladder, bowel, prolapse, and sex dysfunction was 25.63% (41/160), 53.75% (86/160), 1.88% (1.88%), and 23.13% (37/160) respectively. The prevalence of having exactly one, two, and three of the PFD was 47.4%, 35.63%, and 4.38% respectively. The prevalence of bladder-bowel and bladder-bowel-sexual PFD were 15.63% and 2.5% while the prevalence of having at least one (any) of the PFD is 73.1% (Fig. 3).
Distribution of symptom scores for pelvic floor dysfunction. Freq Frequency of daily urination, UI Urge incontinence, SI Stress incontinence, FI Flatal incontinence, DFI Diarrhea fecal incontinence, NSFI Normal stool fecal incontinence, OD Obstructed defecation, VP Vaginal pressure, RV Reduction to void, RD Reduction to defecate, VL Vaginal laxity, Vaginal tightness VT
The multi-variable logistic regression revealed that level of study (years in the university), height, history of UTI, and no sports activity were statistically significant (all p < 0.05) with any (at least one) PFD with an odd ratio of 4.91, > 100, 8.47, and 2.86 respectively. Bladder dysfunction risk factors were level of study and no sports involvement. Bowel dysfunction risk factors were height and sexual dysfunction were history of UTI and no sports activities (Table 5).
Discussion
The burden of pelvic floor disorders affects every area of a woman’s life. Pelvic floor disorders (PFD) can result in multiple adverse outcomes in the daily life of a woman as the majority of women with PFD feel extreme shame, humiliation, and anxiety because of their health status resulting in depression. Women with advanced Pelvic organ prolapse (POP) and Urinary incontinence (UI) have decreased body image and lower quality of life with some isolating themselves from society. PFD also interferes with a woman’s daily activity like sitting down on the toilet, walking long distances, or lifting heavy materials. Perhaps the most distressing effect of PFD is sexual dysfunction which could lead to conflict in their marriages with some of them ending up in divorce. About 67.7% of women with advanced POP are depressed and are also victims of social discrimination when disclosing their health problems [20, 21].
In view of the foregoing, prevention of PFD has become a major priority in women’s health, and identification of women at risk is a key element in current prevention strategies. A prior study reported that one of the major barriers to effective prevention is the inability to effectively identify “at-risk” women [22]. A previous study had identified Urinary incontinence before pregnancy as one of the major risk factors for subsequent pelvic dysfunction [15]. Hence, lifestyle changes with behavioral modifications that can prevent PFD need to be adopted from an early phase of life. Prior evidence would suggest that in some cases, incontinence later in life can either be prevented or the onset delayed with primary prevention and actions taken early, even in the absence of incontinence [15].
The purpose of this study was to determine the prevalence of PFD and associated risk factors in Nulligravida women in order to offer preventative strategies. This is important as even young nulliparas frequently report exercise incontinence with greater prevalence in activities that involve repetitive jumping and bouncing [22]. Therefore, an investigation into the prevalence and risk factors for PFD in Nulligravida women who have never been pregnant will give an insight into the risks in these young women in order to create awareness and also offer preventative measures especially as it regards lifestyle modification. This will greatly minimize the risks of this population of women developing PFD later in life. This is quite pertinent as the prevalence of UI in nulliparous women of childbearing age has been reported to be 10–15% [23] and UI preceding pregnancy in these women has been shown to be a strong indicator for an increased prevalence of urinary incontinence 4–12 years postpartum [24]. The fecal dysfunction section had the highest prevalence of symptoms and the highest occurrence of bother of symptoms among all sections. This finding correlates with the findings in the study by Durnea et al. [1]. It was proposed in that study that the high prevalence of bowel dysfunction symptoms could be partially explained by a high prevalence of pre-existing bowel pathology. However, in this present study, no respondent reported a positive history of pre-existing bowel pathology. The outcome of this study indicated that the majority (64.4%) of the respondents have at least 2 primary symptoms of bladder dysfunction. Stress incontinence was present in 20%, and urge incontinence in 38.8% while urinary urgency (39.4%) was more prevalent than stress incontinence (20%). This finding also correlates with findings from the study by Durnea et al. [1] showing a prevalence of urgency of 42% and stress incontinence of 19.7%. The number of respondents who reported symptoms of pelvic organ prolapse was minimal and this was comparable with results from the NHANES study [25] where a prevalence of 0.6% was reported.
The study by Milsom et al. [6] reported vaginal childbirth as the most probable important factor in the etiology of pelvic floor dysfunction; therefore in our study, the high prevalence of PFD (73.12%) among Nulligravidas who have never been pregnant necessitates further investigations into other factors that may have predisposed this population of women to PFD. Contrary to earlier reports on the association of overweight and obesity with stress incontinence [8], our findings show that BMI had no significant impact and the majority of those with normal BMI (79.1%) had PFD. Age was also not significantly associated with PFD though it was most prevalent in participants in the 20–24 years age bracket contrary to the findings of Ingrid et al. [14] in their study in a population of women in the USA. This is possible because the population in this present study was composed of young women only.
Participation in sports and high-intensity physical activity (PA) among women have been reported as important modifiable risk factors for pelvic floor dysfunction in several studies [26, 27]. The outcome of this study indicates that there is a significant relationship between involvement in sporting activity and the type of sports with PFD in line with a previous study carried out by Giagio et al. [28]. However, logistic regression analysis shows that non-participation in sports was significantly associated with PFD as 68% of those not engaged in sports had PFD. This is contrary to findings from some prior studies that reported a significant impact of sports participation and frequency on PFD as shown by a prior study that concluded that all women who do high-impact exercises are susceptible to stress urinary incontinence (SUI) [27, 29].
The information on sports participation collected from the respondents in this study was limited to conventional recreational sports participation. However, physical activity has been described as not been limited to sports and exercise only but also to activities that occur during work, childcare, eldercare, and housework which are all particularly relevant to women [30]. In their study, by including only recreational physical activity as is commonly done, only 26% of 440 women studied met the CDC guidelines for sufficient activity. However, this proportion increased to nearly 74% when activity from all domains was included [30]. This could be the reason why such a large percentage of participants (68%) in our study who did not engage in conventional recreational sports activities had PFD. In a review of risk factors for pelvic organ prolapse (POP) in developing countries, heavy work, and poor nutrition were associated with POP [29]. Heavy physical work and heavy weight lifting increase intra-abdominal pressure which is believed to play a role in the pathogenesis of PFD, specifically POP [31]. There are scant data on whether strenuous activity when young increases the risk of POP later in life. In a cross-sectional study, it was reported that middle-aged women who reported 21 h/week or more of strenuous activity during their teenage years were more likely to demonstrate POP [25]. In our study, there was no information gathered on the involvement of the participants in heavy strenuous activities such as lifting. This also could have been a contributory factor to the high prevalence observed, though the fact that the participants were college students in a medical school makes heavy lifting very unlikely. It is however important to counsel young girls on the dangers of strenuous work and lifting heavy loads as it has been suggested that dramatic changes in hormones, muscle and bone structure, and weight in the teen years may increase their risk of connective tissue injury which may affect future pelvic floor function [32].
Our results show that a history of urinary tract infection (UTI) was a significant (p = 0.013) risk factor for PFD as 32 (20%) of the participants reported a positive history of UTI. Though PFD is usually due to weak muscles, there is a type of PFD that occurs when the muscles are tight and overactive as it happens when there is an infection. With overactivity, the muscles become shortened, inflexible, and disrupt normal bladder, bowel, and sexual function leading to symptoms such as pain around the vulva and urethra, urinary frequency and urgency, as well as pain with urination [33]. Other symptoms of an overactive pelvic floor include constipation, pain with sex, or even bladder leakage [33]. This could be the possible reason for the significant association seen.
Interestingly, the number of years spent in the university was significantly (p = 0.03) associated with the prevalence of PFD with the majority of the participants being in the 1st and 4th years (32% and 33% respectively) though the reason for this is not really understood. Another significant factor was the height of the participants. A previous study had reported that a maternal height of < 160 cm and a weight of baby > 4 kg were significant risks for PFD [34]. However, the mean height of the participants in our study was 1.65 cm but since they all were Nulligravida, there could be other associated factors responsible for the significant association seen and this would necessitate further investigations.
Another possible reason for the high prevalence of PFD in this population of young women is Childhood nocturnal enuresis (CNE). In a study conducted on nulliparous women, CNE was a significant predictor of a wide range of PFDs, indicating the possible existence of at least one common causal factor linking CNE to later PFDs in nulliparas aged 25–64 years [35]. Though the history of CNE was not obtained from the participants in our study, this could also have been a factor to consider and further studies need to be done to ascertain the association. It has also been established that some Endocrinopathies are related to the reduction of hormonal levels while others are related to an increase [36]. Thus, women with premature ovarian insufficiency (POI) characterized by hypoestrogenism and increased FSH levels and women with hyperandrogenism or other endocrine diseases such as hyperprolactinemia, may present different local hormone levels, though it is not certain if this hormonal micro-environment influences the female pelvic floor [36]. However, in a prior study, though not statistically significant, women with POI presented higher UI prevalence than control women and this may present a good model to understand whether hypoestrogenism may cause pelvic floor dysfunction [37]. On the other hand, however, it has been suggested that the hyperandrogenic state associated with polycystic ovary syndrome (PCOS) which is a heterogeneous disorder affecting young women, is a protective factor for pelvic floor muscles [38].
From the foregoing, it is seen that CNE and Endocrinopathies are other factors that could have been responsible for the high prevalence of PFD seen in this population of Nulligravida young women. In view of this, further studies are necessary to assess the impact of these factors on the pelvic floor so preventive measures against these young women developing PFD later in life can be put in place. These include education and lifestyle modifications such as proper lifting and coughing techniques.
Conclusion
The findings from this study show a high prevalence of fecal and urinary dysfunction, and a low prevalence of sexual dysfunction and pelvic organ prolapse among Nulligravida students, with non-participation in sports and a history of urinary tract infections being the main significant risk factors.
Availability of data and materials
The data for this study are available on request.
Abbreviations
- BMI:
-
Basic metabolic index
- CNE:
-
Childhood nocturnal enuresis
- FD:
-
Fecal dysfunction
- FI:
-
Fecal incontinence (FI)
- FLI:
-
Flatal incontinence
- OAB:
-
Overactive bladder
- OD:
-
Obstructed defecation
- PFD:
-
Pelvic floor dysfunction
- PFM:
-
Pelvic floor muscles
- PCOS:
-
Polycystic ovary syndrome
- POI:
-
Premature ovarian insufficiency
- POP:
-
Pelvic organ prolapse
- SD:
-
Sexual dysfunction
- SUI:
-
Stress urinary incontinence
- UD:
-
Urinary dysfunction
- UI:
-
Urinary incontinence
- UTI:
-
Urinary tract infections
- VL:
-
Vaginal laxity
- VW:
-
Vaginal wind
References
Durnea CM, Khashan AS, Kenny LC, et al. An insight into pelvic floor status in nulliparous women. Int Urogynecol J. 2014;25:337–45.
Al-Badr A, Saleem Z, Kaddour O, et al. Prevalence of pelvic floor dysfunction: a Saudi national survey. BMC Womens Health. 2022;22:27.
Lipschuetz M, Cohen SM, Liebergall-Wischnitzer M, Zbedat K, Hochner-Celnikier D, Lavy Y, et al. Degree of bother from pelvic floor dysfunction in women one year after first delivery. Eur J Obstet Gynecol Reprod Biol. 2015;191:90–4.
Ramaseshan AS, Felton J, Roque D, Rao G, Shipper AG, Sanses TV. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29(4):459–76.
Haylen BT, De Ridder D, Freeman RM, Steven SE, Berghmans B, Lee J, Monga A, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
Milsom I, Altman D, Cartwright R, Lapitan MC, Nelson R, Sillén U, Tikkanen K. Epidemiology of Urinary Incontinence (UI) and other Lower Urinary Tract Symptoms (LUTS), Pelvic Organ Prolapse (POP) and Anal (AI) Incontinence. In: Abrams, Cardozo, Kouhry and Wein, editor. Incontinence. 5th ed. Paris: Health Publications Ltd; 2013. p. 15–107.
Gyhagen M, Bullarbo M, Nielsen T, Milsom I. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. Brit J Obst and Gynec. 2013;120:144–51.
Wing RR, West DS, Grady D, Creasman JM, Richter HE. Effect of weight loss on urinary incontinence in overweight and obese women: results at 12 and 18 months. J Urology. 2010;184:1005–10.
Townsend MK, Danforth KN, Rosner B, Curhan GC, Resnick NM, Grodstein F. Physical activity and incident urinary incontinence in middle-aged women. J Urol. 2008;179(3):1012–6 discussion 1016–1017.
Lawrence JM, Lukacz ES, Liu IL. Pelvic floor disorders, diabetes, and obesity in women: findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007;30:2536–41.
Poylin V, Serrot FJ, Madoff RD. Obesity and bariatric surgery: a systematic review of associations with defecatory dysfunction. Colorectal Dis. 2011;13:e92–103.
Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obs and Gynec. 2007;109:721–7.
Khowailed IA, Pinjuv-Turney J, Lu C, Lee H. Stress incontinence during different high-impact exercises in women: a pilot survey. Int J Environ Res Public Health. 2020;17:8372.
Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obs and Gyne. 2006;194:1455–61.
Ingrid I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J. Prevalence of symptomatic pelvic floor disorders in US Women. J Am Med Ass. 2008;300(11):1311–6.
Sievert KD, Amend B, Toomey PA, Robinson D, Milsom I, Koelbl H, Abrams P, Cardozo L, Wein A, Smith AL, Newman DK. Can we prevent incontinence? ICI-RS 2011. Neurourol Urodynamics. 2012;31:390–9.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Rosenbaum T. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: a literature review. J Sexual Med. 2007;4:4–3.
Baessler K, O’Neill SM, Maher CF, et al. Australian pelvic floor questionnaire: a validated interviewer-administered pelvic floor questionnaire for routine clinic and research. Int Urogynecol J. 2009;20:149–58.
Yount SM. The impact of pelvic floor disorders and pelvic surgery on women’s sexual satisfaction and function. J Midwifery Womens Health. 2013;58(5):538–45 pmid:26055700.
Gjerde JL, Rortveit G, Muleta M, Adefris M, Blystad A. Living with pelvic organ prolapse: voices of women from Amhara region. Ethiopia Intern Urogynecology J. 2017;28(3):361–6 pmid:27475794.
Radoja I, Pavlović O, Perić N, Degmečić D. Coital urinary incontinence and female sexual function. Southeastern Euro Med J. 2017;1(2):11–8.
Fernandes A, Fitz F, Silva A, Filoni E, Filho JM. 0016 Evaluation of the Prevalence of Urinary Incontinence Symptoms in Adolescent Female Soccer Players and their Impact on Quality of Life. Occup Environ Med. 2014;71(Suppl 1):A59-60.
Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric. 2019;22(3):217–22.
Al-Mukhtar Othman J, Åkervall S, Milsom I, et al. Urinary incontinence in nulliparous women aged 25–64 years: a national survey. Am J Obstet Gynecol. 2017;216(149):e1-11.
Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J. Prevalence of symptomatic pelvic floor disorders in US women. J Am Med Assoc. 2018;300(11):1311–6.
Associations NFoSHS. [Accessed July 29, 2015, 2015]; 1969–2014 High School Athletics Participation Survey 2015 http://www.nfhs.org/ParticipationStatics/ParticipationStatics.aspx/
Giagio, Silvia & Salvioli, Stefano & Pillastrini, Paolo & Innocenti, Tiziano. Sport and pelvic floor dysfunction in male and female athletes: a scoping review. Neurourol and Urodynamics.2020; https://doi.org/10.1002/nau.24564.
Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22(2):127–35.
Middlekauff M, Lee W, Egger MJ, Nygaard IE, Shaw JM. Physical activity patterns in healthy middle-aged women. J Women Aging. 2016;28(6):469–76.
Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG. Williams GYNECOLOGY. 2nd ed. China: McGraw-Hill Companies; 2012.
Wild CY, Steele JR, Munro BJ. Why do girls sustain more anterior cruciate ligament injuries than boys?: a review of the changes in estrogen and musculoskeletal structure and function during puberty. Sports Med. 2012;42(9):733–49.
Ashley Rawlins (2022).Your UTI symptoms could be pelvic floor dysfunction in disguise. PELVIC + SEXUAL HEALTH. APR 01, 2022DR. https://www.theoriginway.com/blog/your-uti-symptoms-could-be-pelvic-floor-dysfunction-in-disguise Assessed 19th May, 2023
Gyhagen M, Bullarbo M, Nielsen T, Milsom I. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or cesarean delivery. Brit J Obs and Gynec. 2013;120:144–51.
Othman JA, Åkervall S, Molin M, Gyhagen M. Childhood nocturnal enuresis-a marker for pelvic floor disorders and urinary tract symptoms in women? Int Urogynecol J. 2021;32(2):359–65.
Fante JF, Ferreira CHJ, Juliato CRT, Benetti-Pinto CL, Pereira GMV, Brito LGO. Pelvic floor parameters in women with gynecological endocrinopathies: a systematic review. Rev Assoc Med Bras. 2020;66(12):1742–9.
Tan R, Pu D, Cao J, Ge H, Chang X, Ye G, et al. Prevalence of stress urinary incontinence in women with premature ovarian insufficiency. J Womens Health (Larchmt). 2018;27(12):1508–12.
Micussi MT, Freitas RP, Varella L, Soares EM, Lemos TM, Maranhão TM. Relationship between pelvic floor muscle and hormone levels in polycystic ovary syndrome. Neurourol Urodynam. 2016;35:780–5.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
AIA and DIE designed the concept of the study, including the data collection and data analysis. OJB did the data analysis and statistical analysis; and DIE, JAO, and AIA did the literature search, manuscript preparation, and editing. All authors reviewed and approved the manuscript. AIA is the “guarantor” for this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Institutional Research Ethics Committee of the College of Medicine, University of Lagos before the commencement of the study, and informed consent was sought from all participants.
Consent for publication
All authors reviewed and approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aiyegbusi, A.I., Eze, D.I., Balogun, O.J. et al. Prevalence of pelvic floor dysfunction and associated risk factors among Nulligravida college students: a cross-sectional study. Bull Fac Phys Ther 28, 36 (2023). https://doi.org/10.1186/s43161-023-00147-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43161-023-00147-6