Abstract
Background
Omental cysts are rare benign tumours. They occur due to malformation of the mesenteric lymphatic spaces which fail to communicate with the systemic lymphatic drainage. Diagnosis is challenging due to its rarity, indolent clinical progress, and non-specific clinical presentation in a normally well child. This case highlights the important clinical and radiological features of a giant omental cyst and a different perspective of management using combined ultrasound-guided percutaneous drainage with laparoscopic surgical excision.
Case presentation
We report a case of a giant omental cyst in a 3-year-old boy who presented with gradual abdominal distension over 3 months. He was initially treated for constipation. He had no abdominal pain or history of trauma. There was a non-shifting dullness and fluid thrill on abdominal examination. Persistent distention led to further imaging with ultrasound and computer tomography scan which revealed a giant cystic lesion occupying the whole abdominal cavity. A diagnosis of a giant omental cyst was made. The initial ultrasound-guided percutaneous drainage with gradual decompression of the cyst facilitated a safe and complete laparoscopic excision of the cyst. He recovered well after surgery.
Conclusion
Attention to important clinical and radiological features helps in the diagnosis of a giant omental cyst. Management with combined ultrasound-guided pigtail drainage and laparoscopic excision is safe and feasible.
Similar content being viewed by others
Background
Omental cysts are a very rare entity. Gairdner first described an omental cyst in 1852. In 1880, Tillaux successfully performed the first operation on a mesenteric cyst, and in 1883, Pean reported the first successful marsupialization of a mesenteric cyst [1].
The reported incidence of mesenteric and omental cysts is 1 per 20,000 hospital admissions in children, and only 2.2% consists of omental cysts. The mean age of presentation is 4.5 years with 15% presenting under the age of 10 years [2, 3].
The most common origin of these cysts is lymphangioma [4]. Small cysts can be an incidental finding in an asymptomatic patient. Large cysts usually present with abdominal pain, abdominal distension, palpable mass, nausea, and vomiting [5].
Treatment requires complete surgical excision. Laparoscopic excision is challenging in large omental cysts. The initial ultrasound-guided percutaneous drainage followed by laparoscopic excision allows for a safe and complete excision.
Case presentation
A 3-year-old boy was seen at a local community clinic for abdominal distension and constipation. He was given laxatives and regular follow-up to monitor his symptoms. He was a well and active child with no medical problems. There was no history of trauma. His appetite was normal, and his growth and development were appropriate for his age. The abdominal distension was gradual over 3 months, during which time there was a notable weight loss (15 to 12.5 kg) due to reduced oral intake. He was referred to our centre for further management.
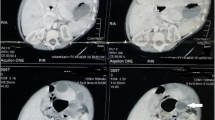
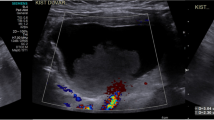
Physical examination revealed a generally well child, less active due to the recent weight loss. His abdomen was grossly distended with visible dilated veins. The abdomen was non-tender, dull (non-shifting) on percussion with the presence of fluid thrill. Laboratory tests included a complete blood count, renal profile, and liver function test which were all within normal range. Initial abdominal ultrasound was suggestive of gross intraperitoneal fluid with multiple septations and internal debris occupying the entire abdomen and pelvis, consistent with complex ascites (Fig. 1). Contrast-enhanced computer tomography (CT) of the abdomen confirmed the findings of a huge intraperitoneal fluid collection with minimal enhancing internal septations. It is located anteriorly displacing both kidneys and bowel loops posteriorly. No intralesional solid component or calcification was seen. There was no peritoneal thickening, nodularity, or enhancement to suggest tuberculous peritonitis. The rest of the abdomen and pelvis were unremarkable. These findings were in favour of a huge intraabdominal cystic mass possibly omental in origin (Fig. 2).
Axial (A), sagittal (B), and coronal (C) images of contrasted CT abdomen and pelvis demonstrates a huge intraperitoneal fluid collection with internal septations (black arrow), located anteriorly and causing mass effect and posterior displacement of the bowel loops (white arrow) and kidneys indicating a space-occupying intraabdominal cystic lesion arising from the omentum
In view of a grossly distended abdomen, a decision was made for gradual drainage. He underwent ultrasound-guided percutaneous pigtail drainage (size 10 Fr) which was placed through his left flank. The pigtail catheter was connected to a urine bag via a three-way tap for controlled drainage of the cyst. Approximately 300 mL (20 mL/kg/day) of straw-coloured cystic fluid was drained daily over a period of 3 days until the abdomen was decompressed. The volume of fluid drained was replaced with an equal amount of intravenous Hartmann’s solution, and daily urine output was closely monitored. Peritoneal fluid analysis showed an exudative picture with a lactate dehydrogenase level of 1198 U/L and a total protein of 44 g/L. Fluid culture and gram stain revealed few pus cells with no organisms seen. Acid fast bacilli stain for Mycobacterium tuberculosis was negative. Peritoneal fluid cytology was negative for malignant cells.
He subsequently underwent laparoscopic excision of the omental cyst. The patient was positioned in lithotomy position with the surgeon standing at the foot-end of the patient and the monitor placed at the head-end of the patient. A 5-mm camera port was placed using the open method at the supraumbilical region, a 3-mm working port was placed on the right abdomen, and a 5-mm working port was placed on the left abdomen. Operative findings revealed a large omental cyst (12.5 × 9.2 × 3.5 cm) arising from the greater omentum. The base of the cyst was along the greater curvature of the stomach. There was no local infiltration to the surrounding structures, and the peritoneal lining was normal. The cyst was already fully decompressed which made the excision feasible laparoscopically without the need for conversion to laparotomy (Fig. 3). The cyst was excised using an ultrasonic energy device.
The surgery and postoperative recovery were uneventful. He was discharged well with no recurrence at 1 year follow-up. Histopathological examination revealed a single-layered cyst wall formed by fibrocollagenous tissue with many lymphatic vessels and lymphoid aggregates. Additional staining for D240 and CD31 was positive and negative for calretinin and BerEP4 which was suggestive of a cystic lesion of lymphatic origin. There was no epithelioid granuloma and ectopic gastric, pancreatic, or bowel tissue.
Discussion
Omental cysts are rare benign tumours. Their true pathoetiology remains unknown. Investigators have postulated that their formation is a result of the absent communication of the embryonic lymphatic spaces with the venous system. Gross proposed a theory of ectopic lymphatics of the mesentery that failed to communicate with the systemic lymphatic drainage [6]. Based on its aetiology, mesenteric cysts can be divided into congenital or embryonic, traumatic, neoplastic, and infectious [7]. Histopathologically, these cysts can be characterised as lymphatic, mesothelial, enteric, urogenital, mature cystic teratoma, and nonpancreatic pseudocyst [8]. Immunohistochemical studies can further differentiate a mesothelial cyst from a lymphatic cyst. Positive stains for calretinin, EMA, and BerEP4 are indicative of mesothelial origin, whereas positive stains for D2-40, CD31, and factor VIII are characteristics of a lymphatic cyst [8, 9].
The clinical presentation of an omental cyst depends on its size, location, and presence or absence of complications [3]. Small cysts are asymptomatic and can be an incidental finding on ultrasound. Large cysts may present with abdominal distension, abdominal pain, nausea and vomiting, a palpable abdominal mass, constipation, or diarrhoea [5]. Abdominal pain may mimic acute appendicitis [10]. Cysts with complications such as infection, mass effect on adjacent structures, haemorrhage, torsion, or rupture present as acute abdomen which necessitate emergent intervention [10]. In the present case, early detection was a challenge due to the non-specific nature of symptoms. Abdominal distension was gradual with no accompanying pain or vomiting. Weight loss may not be apparent and easily overlooked in a well child. Hence, the presence of non-shifting dullness with fluid thrill in a grossly distended abdomen is an important sign to differentiate a large cystic mass from ascites. These clinical findings should be confirmed with early imaging to avoid delays in management.
The ubiquity and easy access to ultrasound make it an important and informative first-line diagnostic modality. Ultrasound may show multiloculated intraperitoneal fluid collection with multiple thin septations. Low-level internal echoes can be seen in an infected or haemorrhagic cyst. A ‘giant’ cystic lesion which occupies the entire abdomen and pelvis can be difficult to differentiate from complex ascites [10]. Additional information regarding the origin of the lesion, its extension, characteristics, and degree of mass effect onto adjacent intraabdominal structures can be obtained through computer tomography (CT) or magnetic resonance imaging (MRI). An important feature that distinguishes an omental cyst from ascites is the presence of mass effect on adjacent structures. In this case, the omental cyst can be seen displacing the bowel loops and kidneys posteriorly. In ascites, free fluid would be seen between the bowel loops, and the bowels would be floating anteriorly within the fluid collection. On MRI, a cyst of lymphatic origin usually demonstrates T1-hypointense and T2-hyperintense signal with no specific findings on diffusion-weighted imaging [11]. Haemorrhagic, proteinaceous, or fatty components within the cyst may affect the MRI signal intensity and CT attenuation. Other differential diagnoses would include pancreatic pseudocyst, ovarian cyst, meconium pseudocyst, choledochal cyst, splenic cyst, intestinal duplication cyst, urachal cyst, and hydrometrocolpos.
Complete excision of the cyst remains the main aim of treatment to avoid recurrences. Excision can be performed via laparotomy or laparoscopy. Laparoscopic excision is preferred in small lesions due to its minimally invasive nature, cosmesis, and early postoperative recovery [12]. In giant omental cysts, laparoscopic excision can be technically challenging with a possibility of cyst rupture or respiratory compromise due to diaphragmatic splinting. Laparoscopic-assisted needle aspiration has been used to decompress large cysts followed by complete laparoscopic excision [13] or extracorporeal excision via an extended umbilical wound [14]. Our method of combined ultrasound-guided pigtail drainage (gradual intermittent) immediately prior to laparoscopic excision, to our knowledge, has not been described in the English literature. Ultrasound-guided pigtail catheter insertion was chosen to safely avoid any abdominal wall or cystic wall vessels. Haemorrhage during insertion may lead to the need for emergency operation. Gradual intermittent drainage of cystic fluid was done to avoid sudden fluid shifts in a small child which may cause hypovolemia [15]. Besides, diaphragmatic splinting can be reduced, and induction of anaesthesia can be given with a lower risk of respiratory compromise. Most importantly, a well-decompressed abdomen allows proper laparoscopic visualisation with adequate working space and accurate identification of intraabdominal structures. Excision of the cyst can be done in a controlled and safe environment. Disadvantages of this method would be a longer hospital stay and risk of infection. This risk can be minimized by adhering to strict aseptic techniques during catheter insertion and connection to a closed drainage system. This method proved successful and safe for our patient.
Conclusion
Giant omental cysts are rare in the paediatric group. Diagnosis and treatment can be challenging. Clinical findings of non-shifting dullness and fluid thrill on abdominal examination should raise the suspicion of a large cystic abdominal mass. Key radiological features of a cystic lesion with mass effect to adjacent structures differentiate a giant omental cyst from ascites. Combined ultrasound-guided pigtail drainage and laparoscopic excision of a giant omental cyst is safe and feasible.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- EMA:
-
Epithelial membrane antigen
References
Vanek VW, Phillips AK. Retroperitoneal, mesenteric, and omental cysts. Arch Surg. 1984;119(7):838–42 1. Available from: https://jamanetwork.com/journals/jamasurgery/fullarticle/590470. Cited 2020 Aug 20.
McMahon SV, McDowell DT, Sweeney B. Mesenteric and omental cysts. In: Pediatric surgery. Berlin: Springer Berlin Heidelberg; 2017. p. 1–6. Available from: http://link.springer.com/10.1007/978-3-642-38482-0_69-1. Cited 2020 Sep 9.
Walker AR, Putnam TC. Omental, mesenteric, and retroperitoneal cysts: a clinical study of 33 new cases. Ann Surg. 1973;178(1):13–9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1355855/. Cited 2020 Sep 27.
Adikibi BT, Wood R, Pillay K, Millar AJW. Omental cyst presenting with profound anaemia. Afr J Paediatr Surg. 2013;10(2):180–4.
Caropreso PR. Mesenteric cysts: a review. Arch Surg. 1974;108(2):242–6. Available from: https://jamanetwork.com/journals/jamasurgery/fullarticle/578595. Cited 2020 Sep 15.
Ricketts RR. Mesenteric and omental cysts. In: Pediatric surgery. 6th ed: Elsevier Inc.; 2006. p. 1399–406.
Beahrs OH, Judd ES, Dockerty MB. Chylous cysts of the abdomen. Surg Clin North Am. 1950;30(4):1081–96.
de Perrot M, Bründler M-A, Tötsch M, Mentha G, Morel P. Mesenteric cysts. Digest Surg. 2000;17(4):323–8. Available from: https://www.karger.com/Article/FullText/18872. Cited 2020 Sep 12.
Suthiwartnarueput W, Kiatipunsodsai S, Kwankua A, Chaumrattanakul U. Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol. 2012;18(43):6328–32.
Shafi S, Malla M, Reshi F. Giant primary omental cyst mimicking a pseudoascites. Afr J Paediatr Surg. 2009;6(1):58. Available from: http://www.afrjpaedsurg.org/text.asp?2009/6/1/58/48581. Cited 2020 Sep 25.
Karhan AN, Soyer T, Gunes A, Talim B, Karnak I, Oguz B, et al. Giant omental cyst (lymphangioma) mimicking ascites and tuberculosis. Iran J Radiol. 2016;13(3):e31943. https://doi.org/10.5812/iranjradiol.31943.
Pampal A, Yagmurlu A. Successful laparoscopic removal of mesenteric and omental cysts in toddlers: 3 cases with a literature review. Journal of Pediatric Surgery. 2012;47(8):e5.
de Lagausie P, Bonnard A, Berrebi D, Lepretre O, Statopoulos L, Delarue A, et al. Abdominal lymphangiomas in children: interest of the laparoscopic approach. Surg Endosc. 2007;21(7):1153–7. Available from. https://doi.org/10.1007/s00464-006-9091-x.
Al-Zaiem MM. Assisted laparoscopic excision of huge abdominal cysts in newborns and infants using the umbilical laparoscopic port incision. Journal of Pediatric Surgery. 2011;46(7):1459–63.
Kramer RE, Sokol RJ, Yerushalmi B, Liu E, MacKenzie T, Hoffenberg EJ, et al. Large-volume paracentesis in the management of ascites in children. J Pediatr Gastroenterol Nutr. 2001;33(3):245–9.
Acknowledgements
Nil
Funding
Nil
Author information
Authors and Affiliations
Contributions
THH: conception, design of the work, acquisition, analysis of the data, and preparation of the manuscript. TSK: acquisition and analysis of the data, drafting, and critical review. NFSNM: analysis of the data and critical review. MAN: conception, analysis of data, and critical review. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written consent had been obtained from the parent of the study participant.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, H.H., Tan, S.K., Nik Malek, N.F.S. et al. Clinical and radiological characteristics and considerations in the surgical management of a giant omental cyst: a case report. Ann Pediatr Surg 18, 41 (2022). https://doi.org/10.1186/s43159-022-00178-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-022-00178-z